News

05 April 2021
New year brings new grant announcements for Stowers researchers
Several Stowers researchers have received funding notifications during the first quarter of 2021, including both investigators and trainees.
Read Article
M.S., Biology, National Institute of Agriculture Technology, Biotechnology Institute and University of Buenos Aires
Ph.D., Biology, National Institute of Agriculture Technology, Biotechnology Institute and University of Buenos Aires

We don’t just do science that we can do or that we feel comfortable doing. It’s about doing the science that we want to do.
Research Areas
Development and Regeneration, Genetics and Genomics, Molecular and Cell Biology
Courses Taught
Gene Expression: Transcription to translation; Laboratory Rotation; Thesis laboratory
Honors
2019-2021
Pew Innovation Fund
Ariel Bazzini, Ph.D., joined the Stowers Institute in 2016. His research focuses on gene regulation in development and disease and in unraveling the intricacies in RNA stability and translation.
Bazzini earned a Ph.D. in biology from the National Institute of Agriculture Technology, Biotechnology Institute and University of Buenos Aires. As a doctoral student studying molecular biology, he was fascinated by RNA, and especially messenger RNA (mRNA), a molecule well-known for translating the DNA code into the amino acids that make up proteins.
Bazzini first studied RNA in depth while investigating how viruses affect gene expression in plants during his doctoral studies at the Institute of Biotechnology in Argentina’s National Institute of Agricultural Technology and the University of Buenos Aires. To expand expertise in animal genetics, he took a postdoctoral fellowship position at Yale University in the lab of Antonio Giraldez, Ph.D., who was researching microRNA in zebrafish. Bazzini successfully adapted the ribosome profiling technique (for the first time in a whole embryo) to measure translation in vivo to understand the large range of translation levels between mRNAs in a cell. He initially used this state-of-the-art technique to demonstrate that miRNAs sequentially prevent translation and subsequently RNA decay during embryogenesis.
Using ribosome profiling to define the coding region of the entire zebrafish transcriptome enabled identification of small, translated coding regions in 5’ and 3’ “untranslated regions” (UTRs), as well as in genes that were annotated as non-coding. Later, he showed that coding regions in the 5’UTR are translational repressors.
Bazzini is also interested in understanding what defines the stability of the mRNAs. He discovered that codons, the three-nucleotide ‘words’ read by ribosomes, have a strong effect on the stability of maternal mRNA and thus contain cis-regulatory information that extends far beyond the amino acids they encode. This represents a paradigm shift in how mRNA stability is viewed.
News

05 April 2021
Several Stowers researchers have received funding notifications during the first quarter of 2021, including both investigators and trainees.
Read Article
Press Release
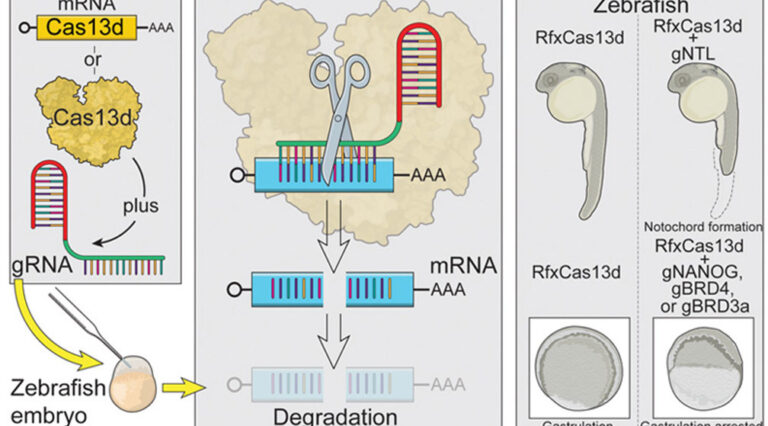
07 August 2020
Scientists in the Bazzini Lab and collaborators have harnessed CRISPR technology to target gene messages in animal model embryos to gain a better understanding of the early stages of vertebrate development.
Read Article
Crosstalk between codon optimality and cis-regulatory elements dictates mRNA stability
Medina-Munoz SG, Kushawah G, Castellano LA, Diez M, DeVore ML, Salazar MJB, Bazzini AA. [published ahead of print January 7 2021]. Genome Biol. 2021;22:14. doi: 10.1186/s13059-020-02251-5.
Translation of small downstream ORFs enhances translation of canonical main open reading frames
Wu Q, Wright M, Gogol MM, Bradford WD, Zhang N, Bazzini AA. EMBO J. 2020;39:e104763. doi: 104710../embj.2020104763.
CRISPR-Cas13d Induces Efficient mRNA Knockdown in Animal Embryos
Kushawah G, Hernandez-Huertas L, Abugattas-Nunez Del Prado J, Martinez-Morales JR, DeVore ML, Hassan H, Moreno-Sanchez I, Tomas-Gallardo L, Diaz-Moscoso A, Monges DE, Guelfo JR, Theune WC, Brannan EO, Wang W, Corbin TJ, Moran AM, Sanchez Alvarado A, Malaga-Trillo E, Takacs CM, Bazzini AA, Moreno-Mateos MA. Dev Cell. 2020;54:805-817 e807.
Brd4 and P300 confer transcriptional competency during zygotic genome activation
Chan SH, Tang Y, Miao L, Darwich-Codore H, Vejnar CE, Beaudoin JD, Musaev D, Fernandez JP, Benitez MDJ, Bazzini AA, Moreno-Mateos MA, Giraldez AJ. Dev Cell. 2019 Jun 17;49(6):867-881.e8. doi: 10.1016/j.devcel.2019.05.037. PMID: 31211993.
Poly(A) tails: longer is not always better
Castellano LA, Bazzini AA. Nat Struct Mol Biol. 2017;24:1010-1011.
Systems to study codon effect on post-transcriptional regulation of gene expression
Wu Q, Bazzini AA. Methods. 2018;137:82-89.
