News

22 June 2022
The switch-like nature of the immune system
Immune cells respond to danger in an “all-or-none” fashion via protein aggregation
Read Article
News
Stowers scientists are unraveling the mechanisms governing cancer stem cells

Resembling a robin’s nest, a cultured mouse intestinal mini-tumor organoid imaged via scanning electron microscopy contains surviving tumor cells (blue) while other tumor cells (brown) die in response to chemoradiotherapy. The nest-like microenvironment forms an immunosuppressive niche that protects cancer stem cells.
By Rachel Scanza, Ph.D.
In 1971, President Nixon declared war. A war on cancer. Fifty-two years later, the war is still raging, with scientists, clinicians, and patients at the front lines. Among the combatants, Linheng Li, Ph.D., an Investigator at the Stowers Institute for Medical Research and co-leader of the Cancer Biology Research Program at the University of Kansas Cancer Center, is making strides toward understanding fundamental principles that unite the hundreds of different cancers and uncovering the specific mechanisms underlying cancers of the blood and intestinal systems including leukemia, adenocarcinoma, and adenomas.
When Li established his lab at the Institute 22 years ago, his research focus was on understanding stem cell biology, and how it related to cancer for a specific type of cancer stem cell. At the most basic level, a stem cell can be compared to a seed that has the potential to grow into a tree. During development, these early cells (or seeds) dynamically mature into all the various cells, tissues, and organs—or roots, trunk, branches, and leaves—of an animal.
Within adult animals including humans, small populations of stem cells still exist within very specific microenvironments (the niche) similar to soil harboring a seed. The niche serves not only to physically anchor cells in place but to maintain stem cells in their naïve, undifferentiated states. Then, when an appropriate signal is received, the niche allows them to migrate and develop into whichever specialized cell type is needed.
“Previous research on bone marrow transplantation proved that stem cells were present within bone marrow,” said Li. “However, how they can be maintained as seed, or undifferentiated, suggested they must reside in a special physical environment.”
3D view of mouse bone marrow containing blood-forming stem cells (magenta) and associated niche cells (yellow and green).
When genetic mutations occur in native or partially differentiated stem cells, or within their niche, a different type of stem cell emerges: Cancer stem cells. Cancer stem cells acquire normal stem cell capabilities like the ability to self-renew while ignoring proliferation-inhibitory signals from the niche, thus driving cancer initiation, or causing relapse.
In 2003, the Li Lab published a study in parallel with a similar study from Harvard University in Nature that was the first definitive identification and proof of a stem cell niche in mammals. Following the discovery of the niche within bone marrow, further research at the molecular level showed that niche signals governing blood-forming stem cells were in principle shared by those controlling the intestinal stem cell niche.
“The concept of a niche that contains stem cells was first proposed by Dr. Ray Schofield in 1978,” said Li. “It took 25 years of research to identify and verify the actual existence of the stem cell niche.”
The mouse gut is composed of finger-like structures called villi which absorb nutrients. At the bottom of villi are ring-like structures called crypts in which intestinal stem cells reside. Mouse intestinal stem cells (green) are located at the bottom of crypts.
Recent research led by Senior Research Specialist Xi He, Ph.D., from the Li Lab has identified a two-pronged mechanism comprised of a sort of dual pathway and a pathologic niche that features frequent back and forth communication. This system explains how intestinal cancer stem cells evade our body’s natural defense system, the immune system, by instead exploiting it for their benefit.
The first pathway, referred to as immune escape, can be pictured as a camouflage or disguise that cancer stem cells don to “hide” in plain sight. Our immune system produces T cells designed to attack and kill foreign invaders and can recognize and destroy cancer cells. The cancer stem cell costume is an almost taunting T cell evasion technique—you cannot find me so you cannot kill me. The immune escape mechanism was uncovered in a 2020 Nature Cell Biology paper by John Perry, Ph.D., a former postdoctoral fellow in the Li Lab, now an investigator at Children’s Mercy Kansas City.
The second pathway, immunosuppression, also serves as a weaponized niche. Cancer stem cells manipulate their environment, using the body’s immune system as both a weapon and a shield. If T cells can be envisioned as spears, cancerous stem cells build a barrier between their microenvironment and the immune cells programmed to kill them. The research also uncovered that the fortified cancer niche and the immune escape pathway talk to each other. For example, previously camouflaged cancer stem cells that have been “found out” by an armed battalion of T cells can send a distress call to the immunosuppression microenvironment: Send reinforcement!
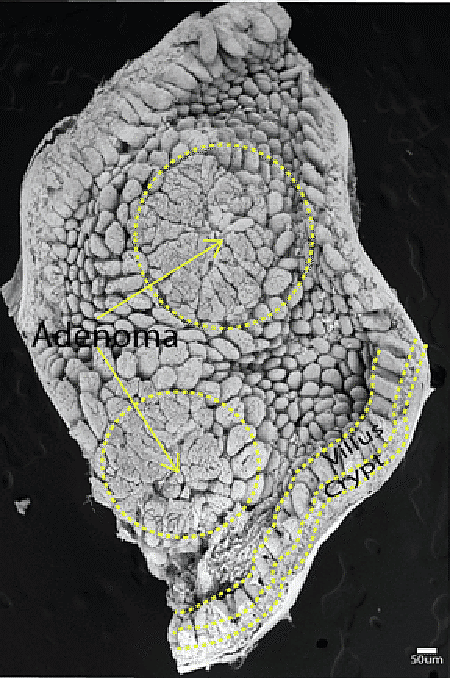
Transmission electron microscopy image of a mouse intestinal adenoma.
The precise pathways for the many variants of cancers may differ. Yet the underlying principle where rogue stem cells use the body’s immune system as both a weapon and a cell phone may eventually lead to discovering new treatments for any type of cancer.
As co-leader of the Cancer Biology Research Program, Li guides the training of the next generation of researchers and physician-scientists who will continue transforming the way we understand and subdue cancer. His role at the KU Cancer Center, one of only 53 National Cancer Institute-designated comprehensive cancer centers in the U.S., also enables him to use insights from his own lab’s research discoveries at the Stowers Institute to support the development of clinical applications.
Analogous to cancer stem cell crosstalk, foundational research can help guide clinical approaches and clinical observations can in turn help guide foundational research. “The benefit of having roles both at Stowers and at the KU Cancer Center allows me to look at fundamental cancer concepts from both angles,” said Li.
Mouse intestinal stem cells (green) sit within cup-like structures that express the cell adhesion molecule N-cadherin (red), forming the niche.
This dual role has led to collaborations with the KU Cancer Center and Children’s Mercy Kansas City. Building on foundational research from the Li Lab that has characterized cancer stem cells at the molecular and cellular levels, these collaborations focus on investigating new approaches for cancer therapies that target and block cancer stem cell pathways, providing hope for patients whose cancer is resistant to drugs.
“Cancer stem cells are smart,” said Li. “They hijack our immune system to promote their own agenda.”
However, researchers are poised to uncover their vulnerabilities. Through foundational and clinical collaboration and with perpetually advancing technologies, scientists and clinicians are understanding more and more about the fundamental principles of cancer development and metastasis. Hope is on the horizon and one day may lead to an armistice or perhaps even a victory in Nixon’s war.
News

22 June 2022
Immune cells respond to danger in an “all-or-none” fashion via protein aggregation
Read Article
News
20 April 2020
The study’s researchers found that low doses of the anthracycline antibiotic doxorubicin inhibit the interaction between two molecular pathways that work closely together to promote tumor growth and resistance to therapy. The targeted approach also clears the way for cancer-targeting immune cells to do their work, an unexpected and novel finding, according to the study authors.
Read Article
Press Release
04 May 2023
Offers unique opportunity to study similar chromosomes linked to cancer and infertility in humans
Read Article
Press Release
15 January 2019
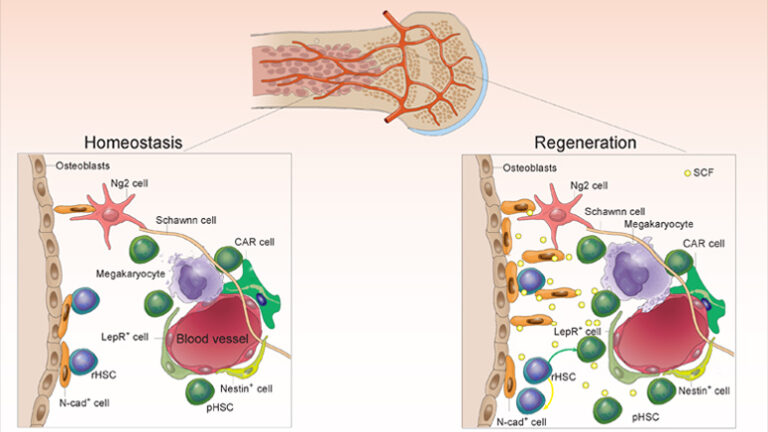
New research from the Stowers Institute for Medical Research has identified a backup for an important biological system – the hematopoietic system, whose adult stem cells constantly replenish the body’s blood supply.
Read Article
