In The News
01 January 2026
Your Cells Are Always Building A Whole New You
From Science Friday, President and CSO Alejandro Sánchez Alvarado talks about the science of regeneration and the biology lessons we can carry into the new year.
Read Article
News
Zulin Yu is a scientist in the Stowers Institute’s Microscopy Technology Center.

What do you think people would find most interesting about your job and the technology you use?
Light microscopy is a valuable tool for examining structures and processes that would otherwise be invisible to the naked eye by using visible light. At the Stowers Institute, researchers use light microscopy to visualize specific structures, proteins, and molecules within tissues and track changes in their behavior over time.
The Stowers Microscopy Center is equipped with a range of cutting-edge light microscopes. As a facility manager and light microscopy scientist, my primary responsibilities include ensuring the facility operates effectively and efficiently. We also offer training to other researchers on how to use the equipment and create educational materials to help them learn more about principles and techniques.
At the Institute, we actively collaborate with lab researchers on various research projects, keep track of the latest developments in the field, and apply them to our facility to stay up to date. This approach can help advance scientific knowledge and discoveries and promote the importance of microscopy and imaging in research.
What exactly is light microscopy?
Primarily, light microscopy utilizes fluorescent dyes or proteins and specialized light sources to visualize samples. In a fluorescence microscope, a specific wavelength of light is used to illuminate the sample, causing the fluorescent molecules to emit light at a different wavelength. Detectors then capture the emitted light, which is used to produce an image of the sample.
The most frequently used fluorescent microscopes at Stowers are laser scanning and spinning disk confocal systems. Laser scanning confocal systems utilize a focused beam of light to scan the sample while pinholes block out-of-focus light, resulting in clearer images. This type of confocal system is particularly useful for studying the 3D structure of biological samples. Spinning disk systems are similar to laser-scanning confocal systems but are 100 times faster. This makes them more suitable for imaging large samples and volumes, and live samples where speed is essential.
Technologies at our center include super-resolution microscopes that can visualize structures even smaller than the wavelength of light.
What does the SIM microscope do?
Conventional light microscopy is limited by the diffraction of light, which restricts the ability to resolve structures smaller than approximately half the wavelength of light used to illuminate the sample (around 200 nm).
To overcome this, super-resolution microscopy techniques have been developed that use a variety of approaches to manipulate the interaction between light and the sample. These techniques include structured illumination microscopy (SIM), stimulated emission depletion (STED) microscopy, and single-molecule localization microscopy (SMLM).
The SIM microscope works by using patterns of light to illuminate the sample. An algorithm is applied to process the resulting images, allowing the SIM microscope to create a high-resolution image of the sample with details beyond the diffraction limit. With SIM, a resolution of up to twice the diffraction limit of conventional light microscopy can be achieved, making it a powerful tool for studying fine details of cells such as microtubules and the nuclear envelope.

Super-resolution microscopy reveals linkages between chromosomes during cell division. These SIM images show cMyc-3 protein labeled in green and UBF protein labeled in magenta, both of which form filamentous chromosomal linkages.
What is the benefit of having this technology at Stowers?
SIM offers several advantages over other super-resolution technologies. Firstly, it is a versatile tool that can work with various conventional fluorescent proteins and dyes, making it suitable for many researchers at Stowers. Additionally, SIM can capture a large field of view simultaneously, making it faster than other super-resolution techniques like STED microscopy. SIM can also be used to image live samples, allowing researchers to study dynamic biological processes in real-time. This makes it particularly useful for investigating cellular processes such as cell division, migration, and signaling.
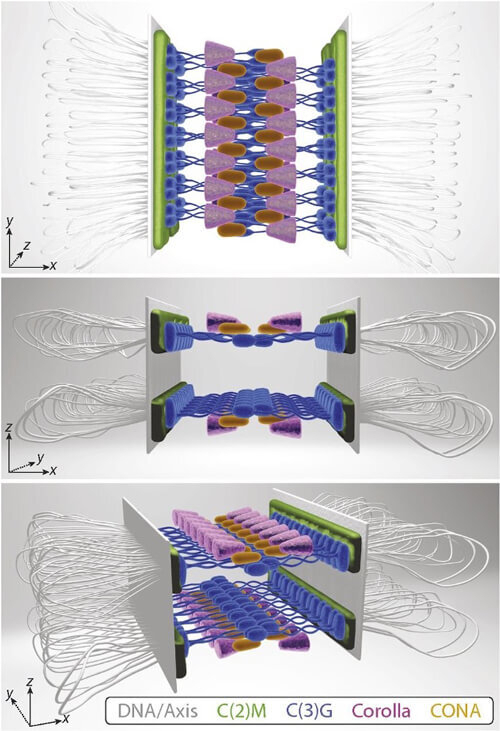
Super-resolution expansion microscopy reveals the structural organization of the fruit fly synaptonemal complex. This 3D model constructed from microscopy data shows two mirrored layers of the synaptonemal complex which is important for meiosis, the cell division that generates eggs and sperm.
What are some of the most exciting discoveries made so far with this technology? Both at Stowers and elsewhere.
SIM has enabled researchers to make many exciting discoveries that were previously impossible with traditional light microscopy since its development two decades ago. According to a PubMed analysis, there have been over 2,000 publications per year in recent years that use SIM, indicating that it has become a widely used tool to answer important questions in biological research.
At the Stowers Institute, Investigator Scott Hawley and his colleagues were the first to use SIM after the technology became available in 2013. They used SIM to image the synaptonemal complex (SC), a protein structure that facilitates the exchange of genetic material between homologous chromosomes during meiosis. The high-resolution images obtained using SIM allowed the researchers to better understand the structure and function of the SC. More recently, the lab presented an updated SC model based on new data from SIM, revealing two layers of SC assembled between homologous sister pairs. SIM has also been used to study a common core configuration of essential centromeric components in human cells by Investigator Jennifer Gerton and her colleagues.
In The News

01 January 2026
From Science Friday, President and CSO Alejandro Sánchez Alvarado talks about the science of regeneration and the biology lessons we can carry into the new year.
Read Article
In The News
26 December 2025
From The Hindu, new research has found that the way the genome is organised can arise from chromatin’s own physics, without needing extra instructions
Read Article
In The News

22 December 2025
From Ophthalmology Breaking News, Stowers scientists study eye regeneration in apple snails
Read Article
