In The News

16 October 2025
AI unlocks the hidden grammar of gene regulation
From ASBMB, the Zeitlinger Lab studies how protein transcription factors, or TFs, bind to DNA to regulate gene expression, using fruit flies as her model.
Read Article
News
Over the ages, these classic song lyrics have taught countless children how their bodies are knitted together. But have you ever wondered why they fit together that way?

By Anissa Anderson Orr
The leg bone’s connected to the knee bone,
The knee bone’s connected to the thigh bone,
The thigh bone’s connected to the hip bone…
Over the ages, these classic song lyrics have taught countless children how their bodies are knitted together. But have you ever wondered why they fit together that way? Or for that matter, why does any organism develop the way that it does—directed according to some unseen plan?
These questions have puzzled scientists for hundreds of years, and it’s only relatively recently, with research into Hox genes, that we are discovering clues to the transformative process that launches an organism’s body plan into motion and sets it in stone.
Researchers at the Stowers Institute are helping lead the way in this exciting field, which is providing new insight not only into how bodies come together, but also on how species evolved. New discoveries also hold promise for understanding human health and disease.
Ironically, while Hox genes are master planners, many important findings in this field have arisen when scientists have gone off plan to investigate a promising idea or unexpected observation, conduct research on a little-studied laboratory animal, or collaborate with colleagues from around the world. The support of institutions like Stowers, which encourages taking risks and providing researchers with the cutting-edge scientific tools and stable financial backing they need to succeed, is critical to making this kind of innovative research happen.
“To me, this is a story about how boldness, creativity, and collaboration can lead to interesting outcomes,” says Stowers Investigator and Scientific Director Robb Krumlauf, PhD.
Unlocking the secrets of Hox Genes
In 1894 scientists made the first observations that eventually led to the discovery of Hox genes nine decades later in 1984, after noticing bizarre transformations in fruit flies (Drosophila) that would be right at home in a horror show. Some had feet where the mouth should be. Others had extra pairs of wings, or legs growing out of their heads instead of antennae. The glitches, called homeotic mutations, were eventually found to be caused by defects in single genes, which the researchers termed homeotic, or Hox, genes.
Research over the next several decades revealed details on how Hox genes control the layout of a developing embryo, marking where structures should appear along the body from head to tail, and determined that the DNA sequences of Hox genes in fruit flies all shared a similar stretch of about 180 bases, called the homeobox. This enabled scientists to find related genes with homeoboxes in other species, including other insects, worms, and even mammals. Hox genes are considered to be a key subset of genes in an organism’s genome that make up the fundamental toolkit needed to control embryonic development. Almost every animal has homeobox sequences in their DNA, so it’s thought that Hox genes emerged early in the evolutionary process.
Krumlauf started investigating Hox genes in the mid-1980s in mice and other vertebrates and has devoted his career to understanding their roles ever since. Amazed by their conserved regulatory powers in so many diverse kinds of animals, Krumlauf says he knew he wanted to spend the rest of his life learning more about these genes and how they serve as master regulatory switches. “As a former chemical engineer, their ability to control biological circuits captivated me. They were part of the instructions for laying down the basic body plan, which I thought was cool.”
Considered a pioneer in the field, Krumlauf was elected to the National Academy of Sciences in 2016 for his seminal work on homeobox genes. He has conducted landmark research on Hox genes in a part of the brainstem called the hindbrain, which controls functions like breathing or blood pressure and makes important contributions to head and face development. He’s also known for co-discovering collinearity of Hox genes, a phenomenon in which the order of these genes in a cluster along a chromosome is the same order in which they’re expressed and function in the animal from head to tail. Over the years, he’s been studying the molecular and cellular pathways that regulate Hox genes in patterning the nervous system and head development and how these processes are linked to human diseases. He has also made evolutionary comparisons of how Hox genes are regulated across species as diverse as mice, chicks, and pufferfish.
Recently, his lab has been focusing on Hox genes in the sea lamprey, an eel-like parasitic fish that is part of a lineage of jawless vertebrates that emerged early in the generation of vertebrates about 550 million years ago. The organism is an important model for studying early events in vertebrate evolution. It’s also an ideal organism in which to explore the mysteries of common toolkit genes and how they are used, which Krumlauf likens to “a wiring diagram,” or an architectural plan used to “put together building blocks and materials to generate very different anatomical structures.”
Very different indeed. How can a similar toolkit of genes drive the development of such a wide variety of species—from the humble fruit fly to much more complex mammals?
To find out, Krumlauf’s lab is exploiting the advantages of the Stowers-built SIMRbase website, a common framework of genomics tools that can be tailored to a wide variety of model organisms. This platform provides a means to systematically compare Hox genes and other regions of the genome across species. SIMRbase enabled Hugo Parker, PhD, a postdoc in the Krumlauf Lab, to publish the identification of lamprey Hox clusters that control head-to-tail patterning, along with other groundbreaking work reporting the germline sequence of the sea lamprey, in Nature Genetics in 2018.
You never know where discoveries will lead. Krumlauf’s studies exploring the signals that control how Hox genes themselves are regulated and expressed in development led to a collaboration with Stowers Investigator Linheng Li, PhD, on the roles of Hox genes in blood cells and cancer. The Krumlauf and Li labs coauthored a study published in Cell Stem Cell in 2018 in which they found that a key regulatory element called DERARE controls the expression of certain Hox genes in blood-forming stem cells. These genes play a critical role in maintaining a healthy balance of blood cells. When they malfunction, they increase the risk for leukemia. Understanding the regulatory mechanisms of Hox genes could help efforts to enhance the function of blood-forming stem cells and find drugs to target certain types of leukemia.
“Hox genes are master regulators. When they go wrong, you may not only end up with abnormal cells or tissues, you could also end up with a disease,” Krumlauf explains.
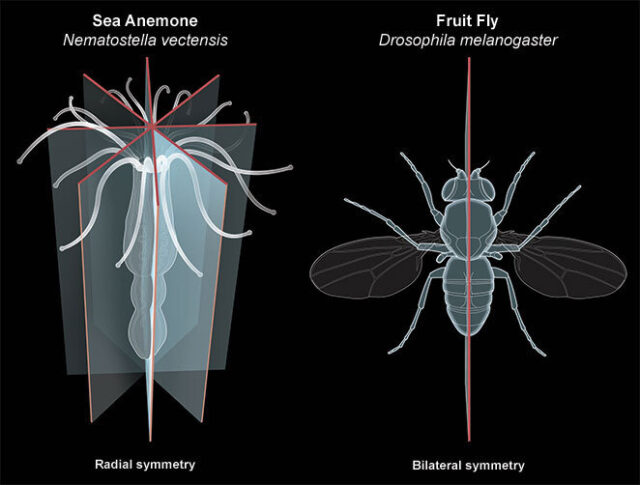
Breakthrough findings in an ancient animal
As much as we’ve learned about the Hox genes, the origin and evolution of this crucial gene family has remained an unsolved mystery. Where did Hox genes come from, and how did their crucial roles in body patterning arise during ancient evolution? This past fall, more interesting discoveries regarding Hox genes emerged from the Stowers Institute—this time from studies on the sea anemone Nematostella vectensis in the lab of Investigator Matt Gibson, PhD.
The role of Hox genes in body patterning had been well-defined in bilaterally symmetric animals like flies, mice, and humans. Surprisingly, these genes are also found in our distant relatives, including simple animals known as Cnidarians (jellyfish, sea anemones and corals). Cnidarians feature radial patterning and lack a head-to-tail axis, raising the question of what Hox genes could be doing. By developing new methods to study developmental gene function in Nematostella, the Gibson Lab found that Hox genes also play an unexpected role in controlling the radially symmetric body plan of Nematostella.
The breakthrough findings, published in Science, were remarkable because Gibson didn’t start his lab at Stowers to investigate Hox genes in sea anemones. He has spent most of his career studying epithelia—highly organized layers of cells that line the internal and external surfaces of the body, such as the epidermis, the outermost layer of the skin. That work takes advantage of the fruit fly, the genetic powerhouse where many Hox genes were initially discovered.
Gibson was drawn to Nematostella because of its large and complex genome, and because it’s flush with epithelial cells. But dedicating part of his research to a much less studied organism was a decidedly risky career move, so Gibson asked for Krumlauf’s input first.
“There was a moment where I saw great potential in Nematostella, but I wasn’t absolutely sure it was a good idea. I asked, ‘Should I really jump into a completely new area?’ And he said, ‘Yes, definitely be bold if that’s what you want to explore.’”
Encouraged by Krumlauf’s enthusiastic support, Gibson ramped up his lab’s focus on the starlet sea anemone. He hired Postdoc Aissam Ikmi, PhD, from the University of Paris, who was willing to take a chance on the new model organism.
“From my perspective, studying different models is like studying different books,” Ikmi says. “Why should we restrict ourselves to studying a few books if we have access to an open library from which we can learn how living organisms solve many problems that matter to our health, as well as other aspects of our lives?”
Ikmi went on to play an essential role in establishing the enormous starlet sea anemone colony needed for research, with support from the Stowers Reptile & Aquatics Facility. He also developed tools, based on cutting-edge gene-editing technologies, to manipulate genes in the sea anemone in collaboration with the Stowers Molecular Biology Facility.
A risk worth taking
Over the course of a decade, the initial risk Gibson took in studying the starlet sea anemone has paid off, yielding several scientific papers and important discoveries, and laying the foundation for the Hox research recently published in Science.
The study’s findings were a major step forward in Hox gene research, because understanding the function of Hox genes in the sea anemone helps us understand their roles in our most ancient common ancestor, some 600 million years in the past.
Using gene knockdown technology, researchers in the Gibson Lab disrupted the function of the genes involved in body patterning of the starlet sea anemone through treatment with short hairpin RNAs, which silence gene function. They also used the CRISPR-Cas9 gene editing approach to remove these Hox genes from the genome.
The results were striking, says Gibson.
“Early in development, Nematostella have a larval stage where the animals look a bit like a little lemon. There’s an outer layer of cells (the rind) covering an inner layer of cells (the fruit). And just like a lemon, when you slice through, Nematostella larvae are divided up into eight segments. When we removed the Hox genes, specific segment boundaries were completely lost. It was a really obvious effect and told us right away that Hox genes control radial segmentation in this animal,” Gibson says. “Furthermore, these segmentation defects led to even more dramatic disruptions of the tentacles that develop right after the larval state.”
In sum, the work shows a key role for Hox genes in radial patterning. The findings were surprising because in bilaterally symmetric animals, Hox genes control the identity of segments along the head-to-tail axis—for example, which part of your spine makes a rib versus a vertebra. In contrast, Hox genes in the sea anemone are responsible for both making and patterning segments.
These functions may have separated over hundreds of millions of years such that Hox genes in present-day bilaterians have lost the role in segment formation and just control segment identity, Gibson says. The finding builds on Krumlauf’s earlier discoveries.
“In the mouse, we showed that a couple of Hox genes work also as segmentation genes, making segments, but their primary role was in segmental identity. People thought that (the finding) was just an odd one-off. But what’s cool about what Matt and his lab have shown is that it may be an ancient feature of Hox genes in making these integrated segmental structures and also giving them an identity. It’s really exciting. It makes everyone reevaluate models on the ancient role of how these systems work,” Krumlauf says.
Training the next generation of Hox researchers
Stowers research trainees have played important roles in advancing Hox gene research.
Ikmi now leads his own lab at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany, where he continues to study Nematostella, and how the interaction between genetic factors and the environment controls developmental processes.
“One key environmental stress is injury, and we focus on understanding the spectacular ability of sea anemones to regenerate—if you cut them in half, each half will grow into a new sea anemone. They’re particularly wellsuited for trying to find out why some animals are better at regeneration than others,” he says.
Working at Stowers gave him the opportunity to develop new technical skills and mature his research plan to help push his scientific knowledge forward, he adds.
Ikmi’s work paved the way for Shuonan He, a predoctoral researcher at the Graduate School of the Stowers Institute for Medical Research and first author of the 2018 Science paper. He joined the Graduate School in 2014, after graduating from Peking University with a bachelor’s degree in biological science. He says his love of marine biology led him to join Gibson’s lab.
“As a mentor, Matt is very open-minded and allows us to develop our own ideas and projects. The freedom to explore is what I enjoy most,” He says. “When I first started in the lab, we already had a well-established, daily-spawning Nematostella colony, thanks to Aissam and the Reptile and Aquatics team, and the CRISPR-Cas gene editing approach had just been demonstrated in Nematostella. I do think it was the perfect opportunity to start my own project on Nematostella.”
His work has centered on generating mutants for different starlet sea anemone Hox genes. The first two years into his project, he had minimal success despite numerous trials. But after a year of exploration and optimization, he was the first researcher to successfully apply short hairpin RNA, or shRNA, to silence gene expression in the starlet sea anemone. The lab now has a much more efficient shRNA protocol that they have shared with many other Cnidarian labs, as well as a more efficient CRISPR-Cas system they used to generate the Hox mutants in the Science paper.
Post publication, He is continuing to focus on the regulatory properties and downstream targets of Nematostella Hox genes. He plans to stay in academia after graduation and continue research in the evolutionary developmental biology field.
What's next?
Scientists at Stowers will continue to dig deeper into Hox gene research, aided by the Institute’s unique research environment which provides access to modern laboratory space, advanced equipment, and scientific support services staffed by expert researchers.
But in addition to equipment and resources, Gibson credits the philosophy of the Stowers Institute, which encourages researchers to take substantial risks to pursue the most interesting ideas. He also points to the support and guiding expertise of Krumlauf, a “phenomenal mentor and a phenomenal human being,” for spurring him to consider taking a different path—one that coincidentally led to breakthrough discoveries about Hox genes that Krumlauf had studied for most of his career.
“When I first came to Stowers, I had no idea that a big part of my lab would wind up working on Nematostella,” Gibson says. “Traditionally, if you set up a fruit fly lab, that’s what you do for 40 years. The support and environment at Stowers really make it possible for scientists to go in new research directions, and that’s a big part of what makes this institution so unique on the world stage.”
In The News

16 October 2025
From ASBMB, the Zeitlinger Lab studies how protein transcription factors, or TFs, bind to DNA to regulate gene expression, using fruit flies as her model.
Read Article
In The News
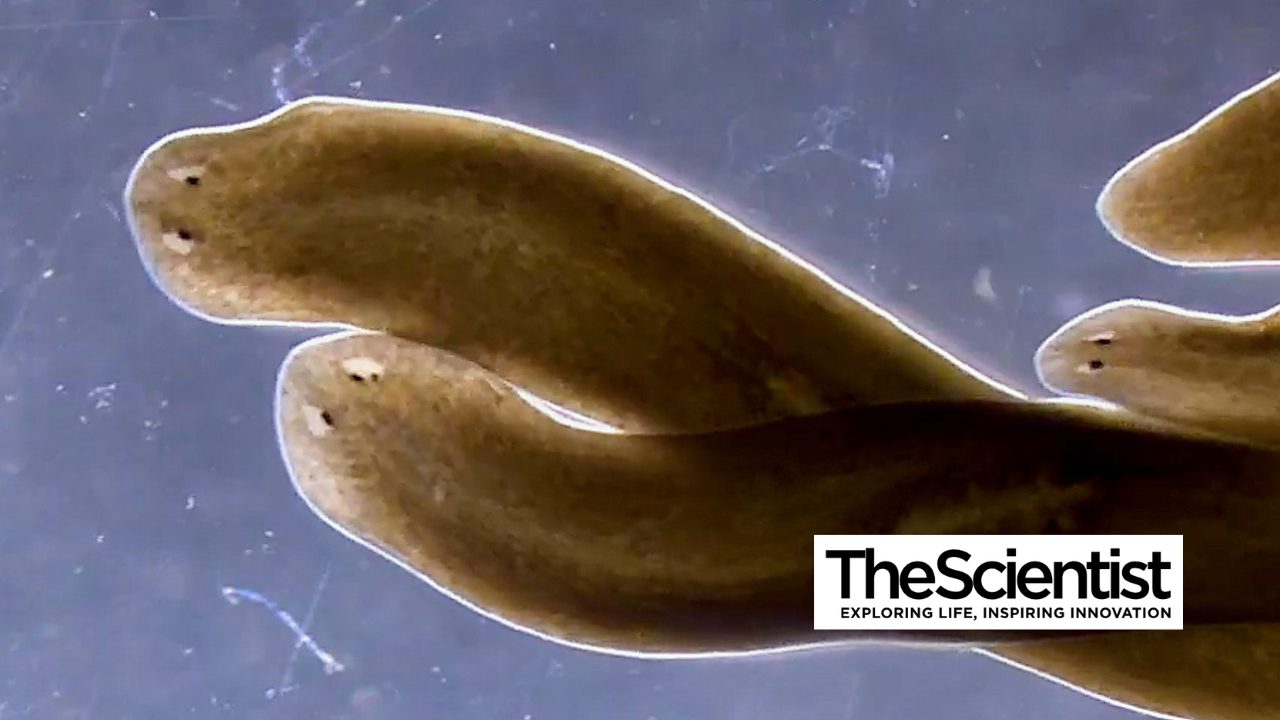
16 October 2025
From The Scientist, research from the Sánchez Alvarado Lab shows stem cells in regenerating planarians don’t need their closest neighbors, overturning researchers’ understanding of the worms’ regenerative superpowers.
Read Article
Press Release
15 October 2025
Stowers scientists discover new rules about how flatworm stem cells regrow body parts, offering insights into potential tissue repair and regenerative medicine in humans.
Read Article
In The News

10 October 2025
From NPR's All Things Considered, in the human body, cells are constantly making life-or-death decisions. If they make the wrong choice, the result can be cancer, infection or even Alzheimer's.
Read Article
