News

18 December 2025
Plants, proteins, and new possibilities for antibiotics and agriculture
Stowers scientist discovers insights into how plants “talk” to bacteria in soil, possibly informing future antimicrobial therapies
Read Article
News

18 December 2025
Stowers scientist discovers insights into how plants “talk” to bacteria in soil, possibly informing future antimicrobial therapies
Read Article
News
17 December 2025
Explore 15 highlights from 2025 at the Stowers Institute: New scientists, impactful discoveries, and a milestone moment.
Read Article

12 December 2025
From El Pais, President and Chief Scientific Officer Alejandro Sánchez Alvarado explains how a tiny animal can teach us how to regenerate organs
Read Article
News
08 December 2025
Craig Venter, Ph.D., founder of the J. Craig Venter Institute, joined Alejandro Sánchez Alvarado, Ph.D., for an evening of reflection and conversation surrounding his scientific journey.
Read Article
In The News

03 December 2025
From KCUR, Stowers researchers are training AI to analyze research data and find the patterns and regulations that make cells function.
Read Article
In The News
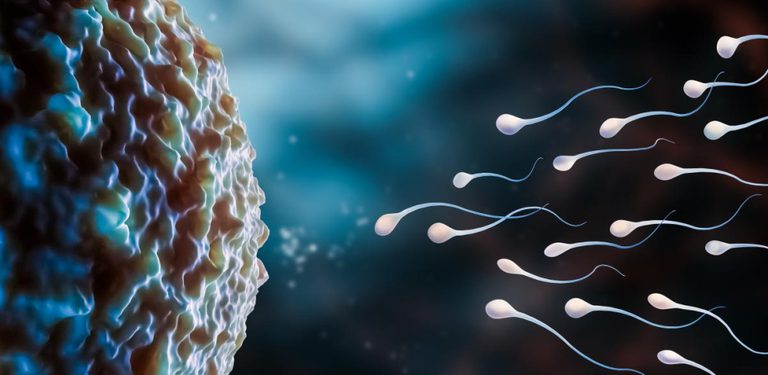
25 November 2025
From La vanguardia, Bazzini Lab study reveals how protein regulation enables embryos to activate their genomes and begin autonomous development in vertebrates.
Read Article
In The News

25 November 2025
From Europa Press, the Bazzini Lab co-led a study identifying a molecular switch that activates the embryonic genome, marking the beginning of autonomous development in vertebrates.
Read Article
In The News
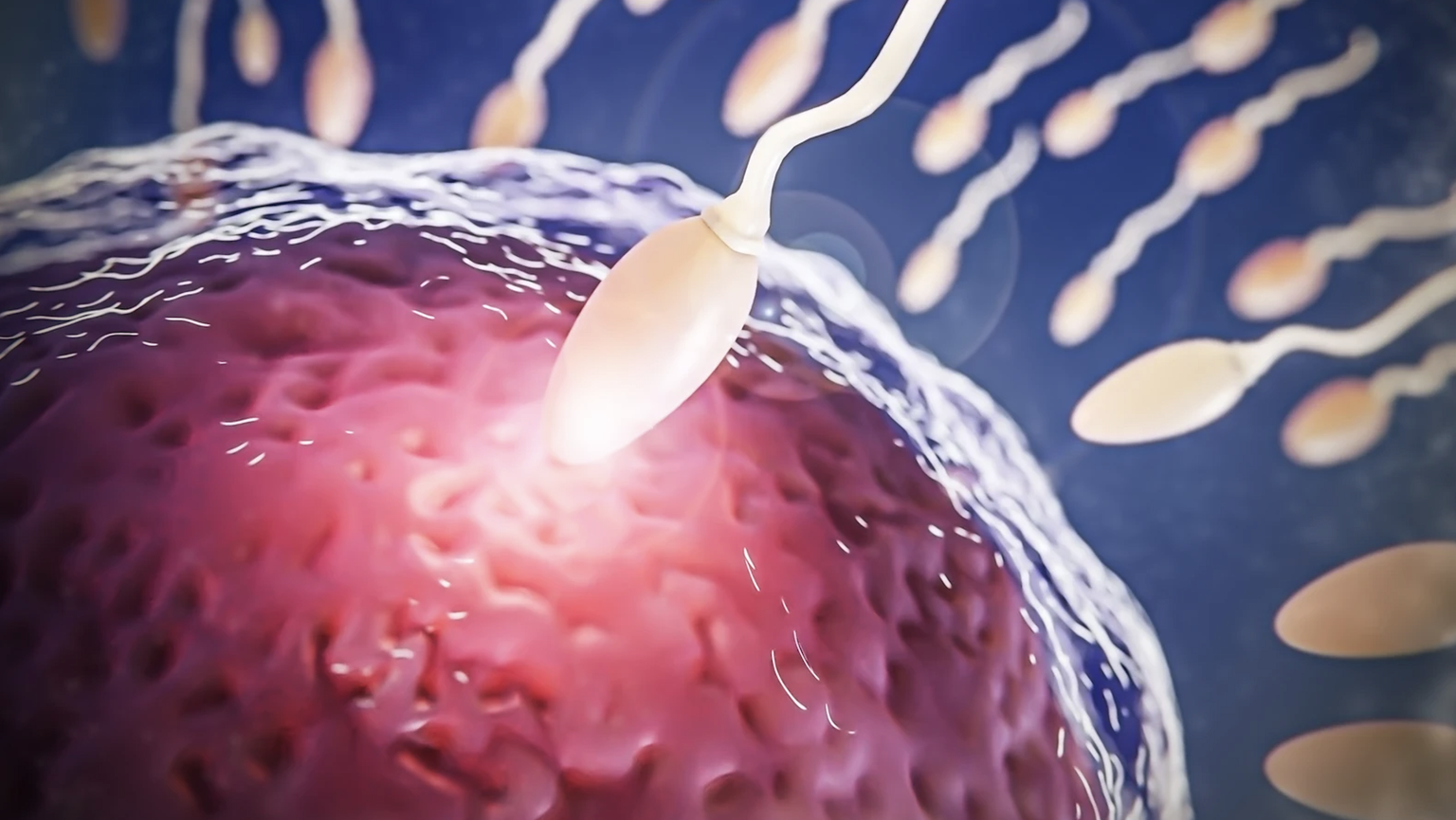
25 November 2025
From Antena 3, the Bazzini Lab has identified for the first time a decisive mechanism that allows the embryo to activate its own genome and autonomously begin its development.
Read Article
#Stowers25: Celebrating 25 Years
24 November 2025
25 Years of Discovery, Innovation, and Hope
Read Article

Browse upcoming talks, seminars, conferences and symposiums, and stay up to date on the latest conferences, speakers, and activities happening at the Institute.

The 2024 Stowers Report celebrates some of the incredible contributions our scientists, students, and staff have achieved this past year, including collaborations, science, and innovations that contribute to our better understanding of the secrets underlying fundamental biological processes.
