In The News
16 May 2024
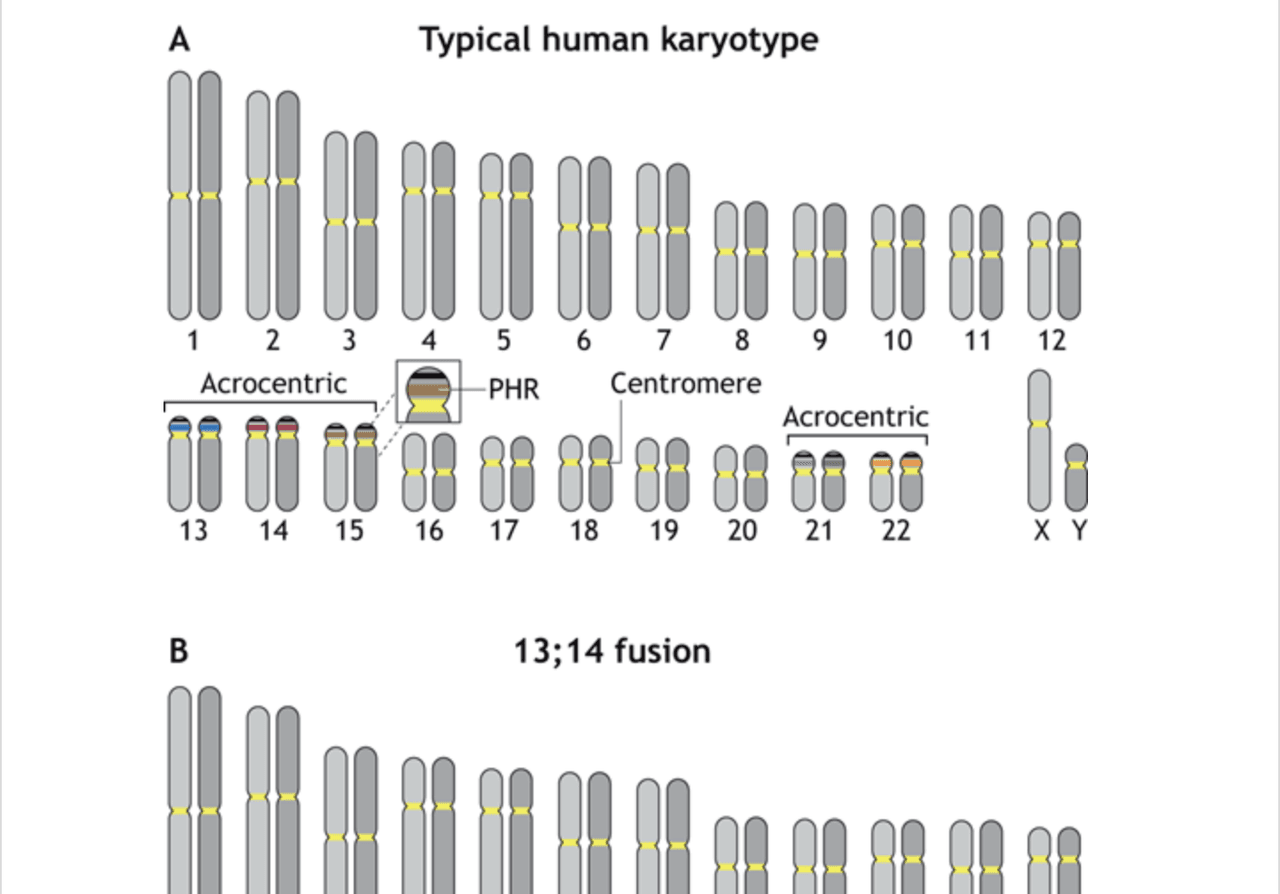
A working model for the formation of Robertsonian chromosomes
From The Journal of Cell Science, read a hypothesis from Stowers Investigator Jennifer Gerton, Ph.D., about the formation of Robertsonian chromosomes.
Read Article
We seek to understand how genome instability impacts cells, tissues, and organisms during development, female reproductive aging, and cancer.
Jump to:
Publications
Research Summary
Genetics and Genomics, Evolutionary Biology, Molecular and Cell Biology
Human cell lines, Yeast, Mice
The Gerton Lab studies chromosomes – the molecular structures made up of DNA and protein that provide the instructions for life. Defects in chromosome function underlie many human diseases such as cancer and birth defects. Using yeast and mammalian model systems as well as human cell lines and patient data, Gerton and her team are investigating groups of proteins responsible for chromosome upkeep.
One group of proteins studied in the Gerton Lab consists of the structural maintenance of chromosome (SMC) proteins. Investigation of cohesin, an SMC complex subtype has provided a wealth of information in its role in chromosome organization.
Gerton initiated and co-organizes the ASBMB special symposium “Emerging roles of the nucleolus” to bring together researchers around this topic. The lab’s research may help provide a better understanding of and potentially new treatment approaches for human conditions such as Roberts Syndrome, Cornelia de Lange Syndrome, and cancers in which cohesin function is impaired.
Gerton has identified several factors that help the kinetochore, another protein complex located on the centromere of chromosomes. Her team is investigating how the loss of chromosome cohesion and other factors impact female reproductive aging.
Principal Investigator
Investigator and Dean of the Graduate School
Stowers Institute for Medical Research
Jennifer Gerton, Ph.D., is a molecular and cellular biologist focusing on chromosome dynamics and behavior during cell division. She joined the Institute in 2002 and currently serves as an Investigator.

Jennifer Gerton, Ph.D., is a molecular and cellular biologist focusing on chromosome dynamics and behavior during cell division. She joined the Institute in 2002 and currently serves as an Investigator.

The Gerton Lab participated in a large international consortium working to complete the assembly of the human genome from telomere-to-telomere. She speculates some newly assembled regions of the human genome may act as phenotypic modifiers of disease states.

Investigator Jennifer Gerton has been at the Institute for 20 years. Listen as she explains why the support here makes it a great place for someone who is just starting a lab.
In The News

16 May 2024
From The Journal of Cell Science, read a hypothesis from Stowers Investigator Jennifer Gerton, Ph.D., about the formation of Robertsonian chromosomes.
Read Article
News

23 August 2023
Will help better our understanding of chromosome structure, evolution, and disease
Read Article
Press Release
23 August 2023
Could potentially improve success rate of cancer drug development
Read Article
A working model for the formation of Robertsonian chromosomes
Gerton JL. J Cell Sci. 2024;137:jcsw261912.
Telomere-to-telomere assembly of a complete human X chromosome
Miga KH, Koren S, Rhie A, Vollger MR, Gershman A, Bzikadze A, Brooks S, Howe E, Porubsky D, Logsdon GA, Schneider VA, Potapova T, Wood J, Chow W, Armstrong J, Fredrickson J, Pak E, Tigyi K, Kremitzki M, Markovic C, Maduro V, Dutra A, Bouffard GG, Chang AM, Hansen NF, Wilfert AB, Thibaud-Nissen F, Schmitt AD, Belton JM, Selvaraj S, Dennis MY, Soto DC, Sahasrabudhe R, Kaya G, Quick J, Loman NJ, Holmes N, Loose M, Surti U, Risques RA, Graves Lindsay TA, Fulton R, Hall I, Paten B, Howe K, Timp W, Young A, Mullikin JC, Pevzner PA, Gerton JL, Sullivan BA, Eichler EE, Phillippy AM. Nature. 2020;585:79-84.
Persistent DNA damage and senescense in the placenta impacts developmental outcomes of embryos
Singh VP, McKinney S, Gerton JL. Dev Cell. 2020;54:333-347 e337.
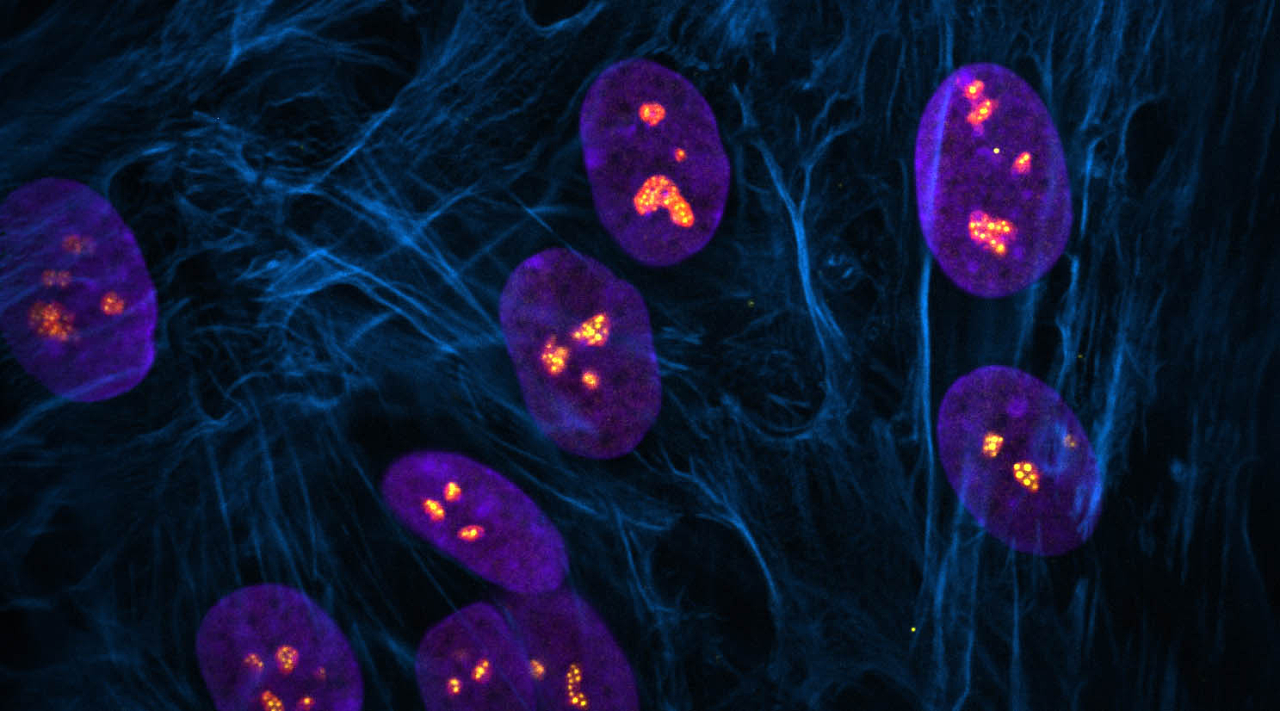
Super-resolution microscopy reveals linkages between ribosomal DNA on heterologous chromosomes
Potapova TA, Unruh JR, Yu Z, Rancati G, Li H, Stampfer MR, Gerton JL. J Cell Biol. 2019;218:2492-2513.
Ribosomal DNA copy number loss and sequence variation in cancer
Xu B, Li H, Perry JM, Singh VP, Unruh J, Yu Z, Zakari M, McDowell W, Li L, Gerton JL. PLoS Genet. 2017;13:e1006771. doi: 10.1371/journal.pgen.1006771
Age-associated dysregulation of protein metabolism in the mammalian oocyte
Duncan FE, Jasti S, Paulson A, Kelsh JM, Fegley B, Gerton JL. Aging Cell. 2017;16:1381-1393.
