Press Release
20 October 2023
Why do some men not produce sperm?
Stowers scientists collaborate to uncover one underlying reason for male infertility
Read Article
Our lab is passionate about understanding all aspects of meiosis, the specialized cell division of sexually reproductive species.
Jump to:
Publications
Click the button below to read more.
Remembering R. Scott Hawley, Ph.D.Research Summary
Genetics and Genomics, Evolutionary Biology, Molecular and Cell Biology
Fruit flies, Silkworms
The Hawley Lab is known for groundbreaking work on meiosis in the fruit fly, Drosophila. Meiosis is a specialized type of cell division required for sexual reproduction. The lab’s studies of egg generation in Drosophila are improving our understanding of how maternal age affects human reproductive capacity due to meiotic anomalies, which often result in miscarriage or birth defects.
Meiosis follows a strict order in which maternal and paternal chromosomes pair up, exchange genetic material, and then separate. Their research addresses: how chromosomes in female fruit flies align and swap genetic information; how they separate into two daughter cells during the first division; and how the second division is coordinated, producing eggs (oocytes) with half the number of chromosomes.
The Hawley Lab has defined factors that govern recombination, a process where chromosomes exchange genetic information. The lab discovered a chromosome-binding protein, Trade Embargo, that governs the first step in initiating recombination. Live imaging is used to observe how chromosomes align as meiosis begins, leading to identification of how the protein Nod nudges chromosomes into proper alignment. The team discovered that the protein, Matrimony, controls the timing of several critical meiotic events by directly blocking the activity of an enzyme controlling meiotic progression.
Principal Investigator
Investigator
Stowers Institute for Medical Research
Scott Hawley, Ph.D., joined the Stowers Institute in 2001, and was an Investigator for the Institute and Dean Emeritus for the Graduate School. Hawley was a noted researcher for his groundbreaking work on meiosis and a dedicated teacher, having trained more than 400 undergraduates over his career.

Scott Hawley, Ph.D., joined the Stowers Institute in 2001, and was an Investigator for the Institute and Dean Emeritus for the Graduate School. Hawley was a noted researcher for his groundbreaking work on meiosis and a dedicated teacher, having trained more than 400 undergraduates over his career.



The lab studies three aspects of chromosome structure and behavior during meiosis: pairing and synapsis, the structure and function of the synaptonemal complex which connects chromosomes, and a fascinating structure known as the recombination nodule. The lab has used the fruit fly Drosophila melanogaster extensively to study these processes. They are also now exploring meiosis in silkworms
Press Release
20 October 2023
Stowers scientists collaborate to uncover one underlying reason for male infertility
Read Article
Press Release
04 May 2023
Offers unique opportunity to study similar chromosomes linked to cancer and infertility in humans
Read Article
News

01 February 2021
The silkworm is one of the most well-studied lepidopteran model systems worldwide, and a recent arrival in the Hawley Lab at the Stowers Institute for Medical Research.
Read Article
News
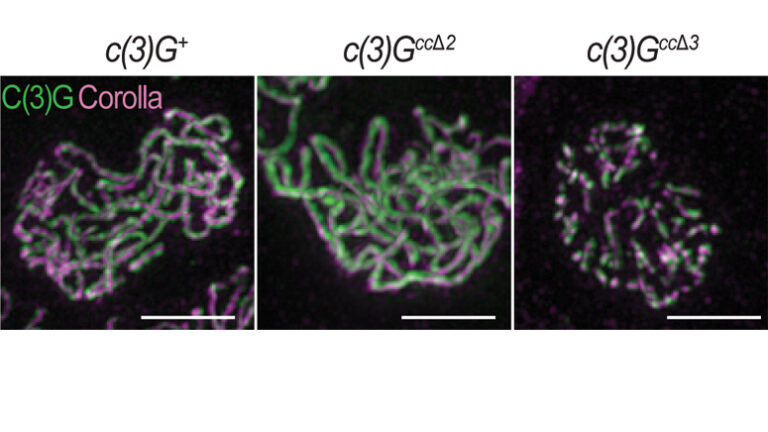
16 October 2019
A recent study from the laboratory of Stowers Investigator Scott Hawley, PhD, has revealed more details about how the synaptonemal complex performs its job, including some surprising subtleties in function.
Read Article
Genetic background impacts the timing of synaptonemal complex breakdown in Drosophila melanogaster
Wesley ER, Hawley RS, Billmyre KK. Chromosoma. 2020;129:243-254.
Miller DE, Kahsai L, Buddika K, Dixon MJ, Kim BY, Calvi BR, Sokol NS, Hawley RS, Cook KR. G3 (Bethesda). 2020;10:4271-4285.
Bonner AM, Hughes SE, Hawley RS. Curr Biol. 2020;30:715-722e713.
Billmyre KK, Cahoon CK, Heenan GM, Wesley ER, Yu Z, Unruh JR, Takeo S, Hawley RS. Proc Natl Acad Sci U S A. 2019;116:21641-21650.
Cahoon CK, Yu Z, Wang Y, Guo F, Unruh JR, Slaughter BD, Hawley RS. Proc Natl Acad Sci U S A. 2017;114:E6857-E6866.
Miller DE, Smith CB, Kazemi NY, Cockrell AJ, Arvanitakas AV, Blumenstiel JP, Jaspersen SL, Hawley RS. Genetics. 2016;203:159-171.
