In The News

07 January 2026
Investigator Kamena Kostova, named ‘Cell Scientist to Watch’
From the Journal of Cell Science, Investigator Kamena Kostova named a 'Cell Scientist to Watch'
Read Article
#Stowers25: Celebrating 25 Years
Highlighting 14 high-impact discoveries from the Stowers Institute's inception
The Stowers Institute campus
By Rachel Scanza, Ph.D.
Scientists at the Stowers Institute have the opportunity to pursue the answers to some of biology’s most complex questions — What is memory? What is the origin of the body’s development? Why do some species regenerate while others do not? As the Institute marks its 25th anniversary, we are highlighting a handful of discoveries that have not only influenced our understanding of fundamental biology but have also improved our knowledge of human health and disease.

Microscopy image of fruit fly ovary where cadherin anchors stem cells in their niche.
Identifying niche anchors
Ting Xie, Science, 2002
Stem cells are maintained by specialized homes called niches, which anchor them and ensure their proper function. In fruit flies, former Investigator Ting Xie, Ph.D., and his group discovered that a protein called DE-cadherin acts like a biological glue to secure ovarian stem cells within their niche. This anchoring depends on direct interactions between DE-cadherin in stem cells and neighboring support cells, with another protein called β-catenin also playing a critical role. When these proteins are missing, the stem cells lose their attachment, drift away, and cannot maintain their stem cell status. These findings revealed that cadherin-based anchoring is vital for healthy stem cell maintenance, a principle that extends across many tissues and species, including humans.
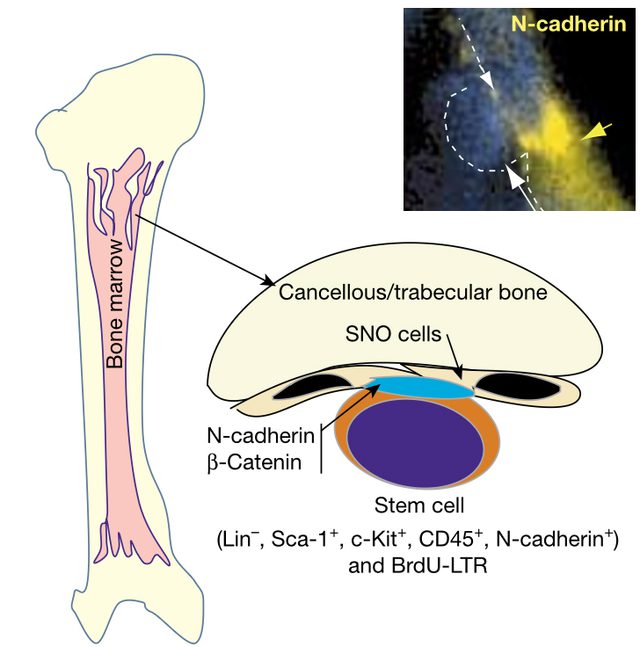
Model illustrating blood stem cell niche with SNO cells as a key niche component. Microscopy image of SNO cells connecting to bone-forming cells (top right).
The niche defines its stem cells
Linheng Li, Nature, 2003
For decades, scientists suspected that adult stem cells (key to renewing blood and gut tissues) needed a dedicated physical niche to control their fate. Investigator Linheng Li, Ph.D.’s landmark 2003 Nature study was the first to pinpoint the precise location of the “stem cell niche” in mouse bone marrow, showing that blood-forming stem cells are anchored to the inner bone surface. Subsequent research used cutting-edge imaging to visualize how hematopoietic (blood) stem cells find and settle within these supportive zones (Nature 2009), revealed how different bone marrow niches can keep stem cells in either a resting or active state (Nature Medicine 2014), and that breakdowns in these zones may spark treatment resistant cancers (Cell Reports 2019; Nature Cell Biology 2021). The concept of stem cell niches defined by their physical environment has transformed how we understand cell renewal and disease.

Graphical illustration of healthy bone (left) and osteoporotic bone (right). Foundational research led to the development of Evenity, the first drug capable of promoting bone regrowth due to osteoporosis.
A “Wise” way for healthy bones
Robb Krumlauf, Development, 2003
In 2003, Investigator and founding Scientific Director Robb Krumlauf, Ph.D., and his group discovered a novel protein called Wise. Wise helps regulate bone formation by controlling the Wnt signaling pathway, a molecular system crucial for both body patterning and bone growth. Their work unexpectedly connected developmental genetics with adult bone health, showing that Wise (and its close relative, sclerostin) restrict bone-building in the skeleton. This foundational research led to the development of Evenity (romosozumab), the first osteoporosis drug proven to substantially increase bone density and prevent fractures by mimicking rare human mutations that block sclerostin. Krumlauf’s findings are now credited with opening new directions for bone therapies benefitting millions of patients globally.
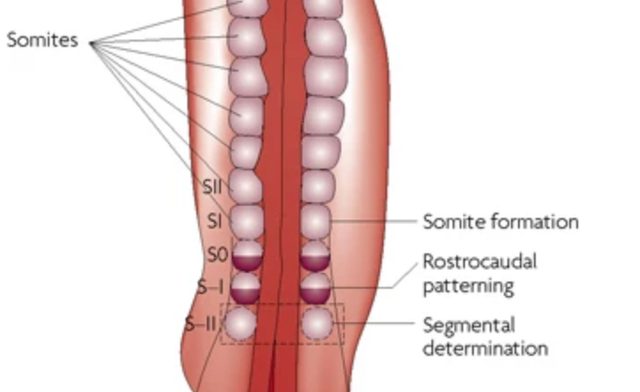
Illustration of segmental patterning of the vertebrate embryonic axis, where somites are blocks of cells laid down in a rhythmic pattern.
Development’s ticking clock
Olivier Pourquié, Science, 2006
Understanding how embryos measure time as they build their bodies has puzzled biologists for decades. Former Investigator Olivier Pourquié, Ph.D.’s, research revealed that embryos use a “segmentation clock,” a cellular rhythm powered by specific genes and signaling pathways, to precisely time the creation of repeated structures such as vertebral segments. His work showed that interconnected signaling systems (including Wnt and Notch) operate together to set this developmental pace, converting the timing of molecular oscillations into spatial patterns that define the backbone. This provided the first clear mechanistic insight into how embryonic cells coordinate complex body structures through temporal and spatial cues.
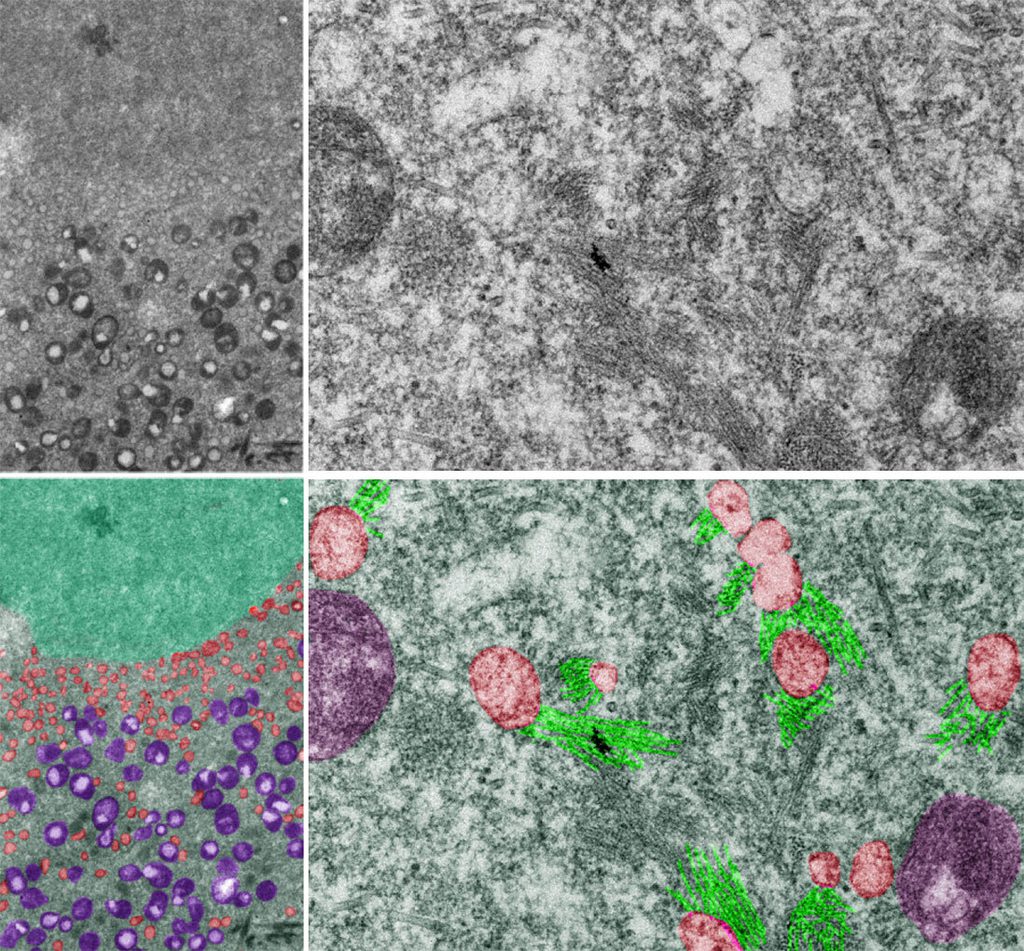
Microscopy image of aneuploid yeast during meiosis.
A stressful advantage
Rong Li, Nature, 2010
While abnormal chromosome numbers (aneuploidy) are typically harmful in complex organisms, former Investigator Rong Li, Ph.D., and her group found that these genetic quirks can actually provide survival advantages in yeast. By engineering yeast cells with different chromosome counts and exposing them to multiple stressors, the researchers discovered that some aneuploid strains thrive under specific stressful conditions. These findings offered fundamental insights into how flexible chromosome numbers can promote adaptive evolution of unicellular organisms and possibly contribute to cancer cells’ survival during chemotherapy in humans, shedding light on the dual nature of aneuploidy in evolution and disease.

The lab grown new species of whiptail lizard, Aspidoscelis neavesi.
Creating a new species
Peter Baumann, PNAS, 2011
Most animal species form when populations are separated and gradually accumulate enough genetic differences to stop interbreeding. But whiptail lizards are different: Some species are made up entirely of females that reproduce without males. Named after Stowers founding President William “Bill” Neaves, a lab grown lizard, Aspidoscelis neavesi, challenged the rules for evolution’s speciation process. Former Investigator Peter Baumann, Ph.D., created a new species of whiptail lizard in the lab, generating a lineage of all-female lizards by hybridization, the crossing of two different species. These lizards reproduced clonally — essentially making genetic copies of themselves — for several generations. The finding showed how hybridization can instantly create new asexual species and provided a powerful model for studying how such unusual lineages evolve.
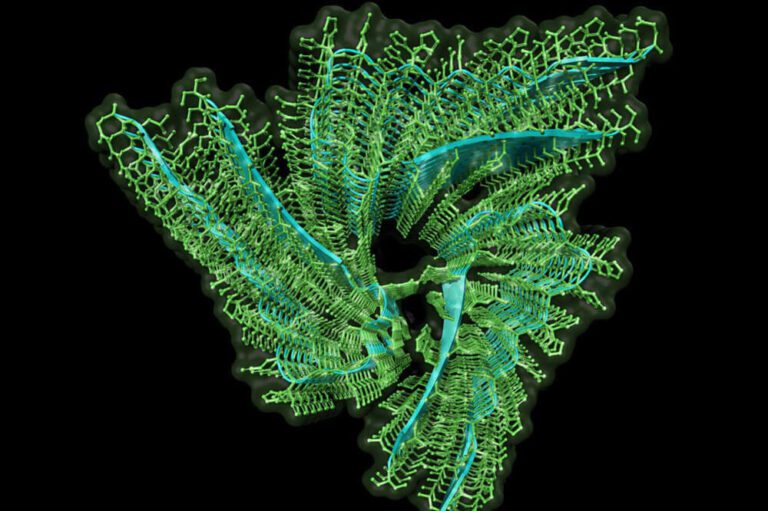
Cryogenic electron microscopy image of the Orb2 amyloid structure responsible for storing memory.
Memories built to last
Kausik Si, Cell, 2012
One of the mysteries of neuroscience is how memories can last a lifetime even though the proteins in our brains are constantly being replaced. Research led by Scientific Director Kausik Si, Ph.D., found the answer in fruit flies — a protein called Orb2 (or CPEB in humans) can form stable, amyloid-like clusters at the connections between neurons. Amyloids are typically considered toxic and associated with debilitating neurodegenerative diseases like Alzheimer’s. However, these clusters act like a lasting "molecular bookmark," preserving the changes in brain cells that store memory. When the team disrupted Orb2’s ability to form amyloids, the flies lost the ability to hold onto memories beyond a day. This finding identified Orb2 as a key player in keeping long-term memories stable.
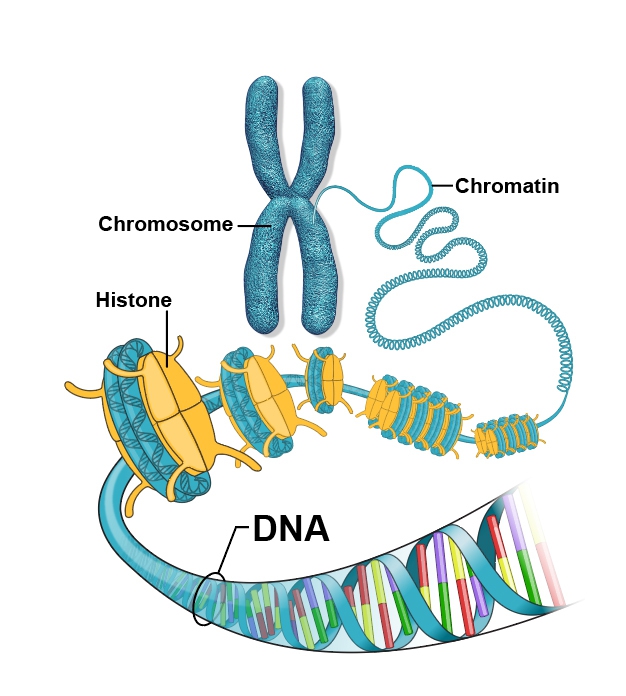
Graphical illustration of the DNA packaging process.
Epigenetics balancing act
Jerry Workman, Nature, 2012
Key proteins that help package DNA called histones can swap or exchange places when needed to make DNA ready to read. DNA winds around histones (akin to thread around a spool) where special proteins can attach chemical “tags” that either tell them to unwind or stay put. The lab of Stowers Investigator Jerry Workman, Ph.D., uncovered how cells maintain genomic integrity by controlling histone swapping during gene transcription. In yeast, the protein Set2 attaches a chemical mark to certain histones, preventing them from being replaced while genes are active. This “locking” mechanism ensures that genes are read accurately, blocking errors that can cause defective proteins or disease. The study explained a previously mysterious safeguard ensuring that only appropriate regions of the genome are dynamically modified, the foundation for healthy gene regulation.
Untangling how DNA packaging drives development
Ali Shilatifard, Science, 2014
To fit inside a cell’s nucleus, DNA is tightly wrapped around proteins called histones, forming a structure known as chromatin. These histones aren’t just packaging — they help control which genes turn on or off. Former Investigator Ali Shilatifard, Ph.D., discovered how mutations in histone proteins (which help package DNA) can disrupt normal switching of gene activity, leading to severe developmental defects (Cell 2007). While at the Stowers Institute, he uncovered that certain histone mutations block these normal switches, causing severe developmental defects in fruit flies (Science 2014). Shilatifard then showed how another protein regulates gene activity by controlling RNA Polymerase II (Pol II), the molecular machine that reads DNA to make RNA (Cell 2015). Because similar mutations in histones and Pol II regulators have been linked to aggressive childhood and adult cancers, Shilatifard’s discoveries are now guiding ongoing clinical trials for novel cancer therapies.
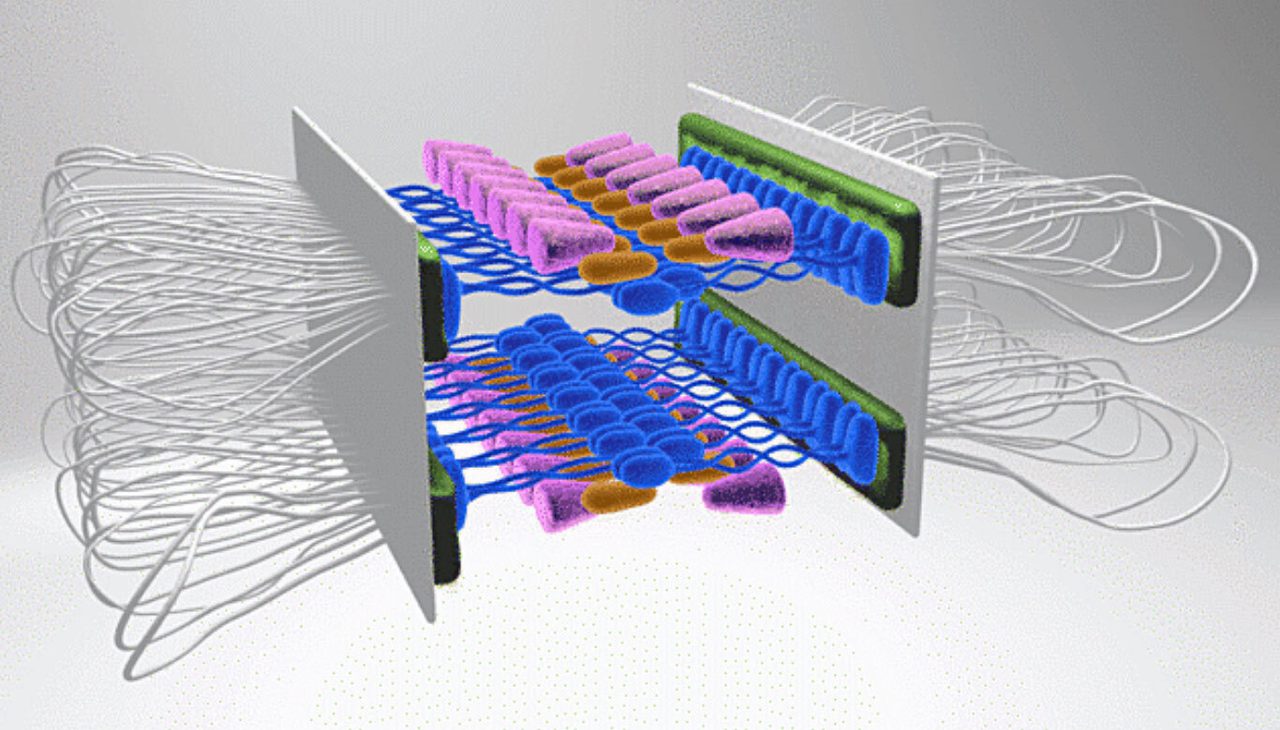
Graphical illustration of the structure of the synaptonemal complex.
Chromosomal choreography
Scott Hawley, PNAS, 2017
Using cutting edge super-resolution and expansion microscopy, the team of former Investigator and founding Dean of the Stowers Graduate School Scott Hawley, Ph.D., mapped the synaptonemal complex (a structure essential for accurate chromosome sorting during the formation of egg and sperm cells) in fruit flies. Its precise structure had remained elusive due to its tiny size; however, Hawley, together with Stowers Microscopy Technology Center scientists, solved a 50-year-old puzzle. Their work produced the first 3D visualization, revealing that the complex consists of two distinct layers, each likely connecting non-sister chromatids. This discovery resolved a decades-old mystery, giving new insight into the molecular choreography that prevents infertility and miscarriage caused by chromosome mis-segregation.

Hox genes in the starlet sea anemone organize internal segments and tentacles.
Master planned
Matt Gibson, Science, 2018
Hox genes, famous for helping animals build their body plans, aren’t just important in creatures with two-sided symmetry like us. Yet how does this “body-building code” work for far more ancient ancestors without an obvious left or right side? Investigator Matt Gibson, Ph.D., asked this very question of the starlet sea anemone, Nematostella, which is a cnidarian, a group of animals including coral and jellyfish. Using modern genetic tools, researchers showed that in sea anemones, hox genes help organize internal segments and tentacle patterns. These findings indicated a sustained evolutionary body planning toolkit dating back to a very early common ancestor of animals, far older than previously thought.
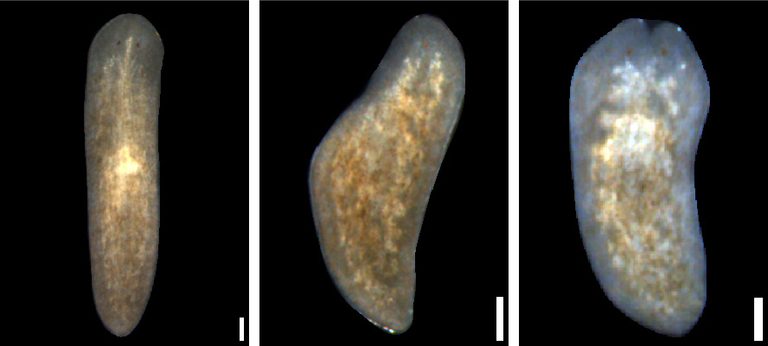
Planarian flatworms where different genes impact regeneration.
Lifesaving cells
Alejandro Sánchez Alvarado, Cell, 2018
The lab of President and Chief Scientific Officer Alejandro Sánchez Alvarado, Ph.D., precisely identified a subset of stem cells in planarians that can regenerate an entire organism from a single cell. Using single-cell analysis and transplantation experiments, Sánchez Alvarado and his team identified a new subtype of stem cell marked by a special protein and showed that these stem cells are truly pluripotent and essential for robust regeneration. This breakthrough enabled scientists to isolate, characterize, and manipulate adult stem cells with unlimited regenerative capability, providing a vital model for stem cell and regenerative biology across all animals.
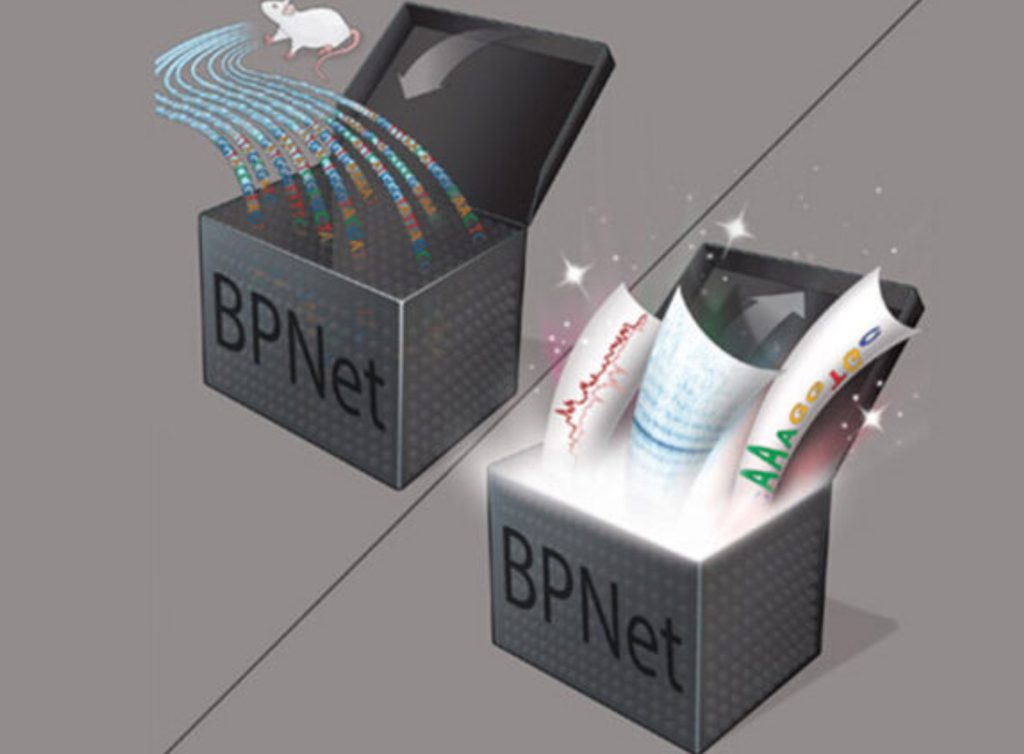
Graphical illustration showing how the AI model BPNet converts DNA sequence to motif predictions.
Decoding DNA with AI
Julia Zeitlinger, Nature Genetics, 2021
One of the big unsolved problems in biology is not just how DNA encodes the instructions from four letters — A, G, T, C — for building proteins but for when and where those proteins are made. The “when” and the “where” are dictated by small stretches of DNA where special proteins bind to signal a chain reaction. These stretches are called motifs, and how they are arranged, and which combinations of proteins must bind at the right time and place is a multi-dimensional problem beyond human scope. This is where AI, or precisely, BPNet (which stands for Base Pair Network), developed by Investigator Julia Zeitlinger, Ph.D., in collaboration with experts from Stanford University truly shines. Zeitlinger’s team trained BPNet on maps of interactions between proteins and DNA, uncovering subtle organizational patterns in sequence that regulate genes — a potential “Holy Grail” for understanding how genes are regulated from DNA sequence alone.
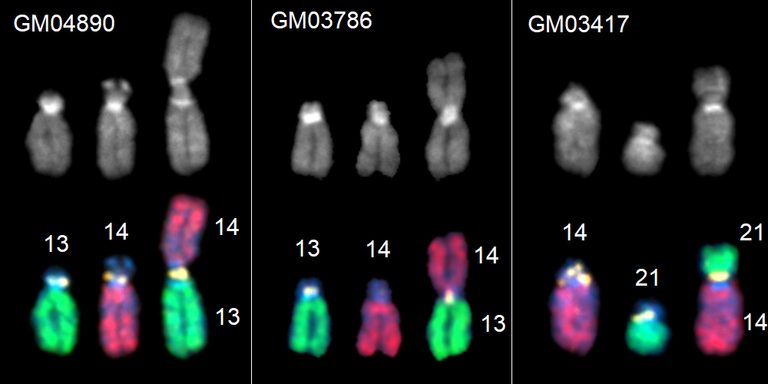
Robertsonian fusion chromosomes and their normal, non-fused counterparts from three different human cell lines.
Illuminating genomic “dark matter”
Jennifer Gerton, Nature, 2025
First observed more than a century ago and the subject of intense scrutiny for 50 years, how exactly Robertsonian chromosomes — two different chromosomes fused together at their centers — arise has remained a mystery. A landmark study from Investigator Jennifer Gerton, Ph.D., solved the puzzle. The researchers identified the precise breakpoint that results in the formation of Robertsonian chromosomes in humans. Because one out of every 800 people have this genetic anomaly, the study is transformative with major implications for understanding infertility, congenital conditions, and chromosome evolution.
Explore all Stowers discoveries over the past 25 years.
In The News

07 January 2026
From the Journal of Cell Science, Investigator Kamena Kostova named a 'Cell Scientist to Watch'
Read Article
#Stowers25: Celebrating 25 Years
06 January 2026
Alejandro Sánchez Alvarado, Ph.D., reflects on a year of discovery, gratitude, and the community that helps support our mission.
Read Article
In The News
01 January 2026
From Science Friday, President and CSO Alejandro Sánchez Alvarado talks about the science of regeneration and the biology lessons we can carry into the new year.
Read Article
