In The News

07 January 2026
Investigator Kamena Kostova, named ‘Cell Scientist to Watch’
From the Journal of Cell Science, Investigator Kamena Kostova named a 'Cell Scientist to Watch'
Read Article
News
Foundational research at the Stowers Institute is leading to a greater understanding of many factors governing infertility with the goal of improving health, healthy outcomes, and providing hope for people facing this challenge.

A fertilized mouse oocyte is captured in the act of completing a cell division, with chromosomes (magenta in the right image) in the center of each newly formed cell.
Many people picture the joy of becoming a parent. Some even fantasize about the names we will give our children, the lessons we want to impart, and all they will accomplish. Add to that our own parent or parents’ expectations of grandchildren. Then, when pregnancy doesn’t happen according to plan and fertility is called into question, the family building process can suddenly become overwhelming, causing feelings of uncertainty, disappointment, and even grief.
According to the Centers for Disease Control and Prevention, infertility rates are rising. A combination of factors may be at play, with more and more couples delaying childbearing. Scientists at the Stowers Institute for Medical Research are investigating infertility from multiple perspectives—genetic, cellular and molecular aging, and evolutionary—to bring hope to people struggling with infertility.
Genetics and age
Infertility is widely defined as a disease of either male or female reproductive systems, or both, if pregnancy is not achieved after 12 months of unprotected sex. An estimated 7% of men and 10% of women are infertile. With thousands of genes involved in male and female reproduction, any number of things can go wrong.
From a genetic perspective, errors in chromosome replication followed by mistakes during sperm and egg production can all result in infertility. Frequently, genetics also underlies structural defects of the reproductive system that impair fertility in both men and women.
Female reproductive aging, a research focus of Stowers Investigator Jennifer Gerton, Ph.D., begins decades before other organ systems start to decline with age. Reproductive function falls precipitously in women by their mid to late thirties before completely ceasing at menopause.
Socioeconomic and lifestyle factors are contributing to many women choosing to have children later in life, and the risk factors for infertility, birth defects, and miscarriages increase with age. Research indicates that a cellular decline in egg quality and not quantity is the primary cause.

The Gerton Lab studies how ovarian tissue and the cells within it change with age.
The Gerton Lab investigates the cellular and molecular mechanisms associated with reproductive aging in women, such as how ovarian tissues change over time, and how this process affects fertility. A long-term goal is to help increase our understanding of reproductive health to help women make more informed health decisions and to identify new ways to improve women’s health and fertility.
Although far more research addresses female infertility, there is growing evidence of a paternal age factor associated with offspring having birth defects or cognitive conditions. Because procreation, at least in humans, takes two, fertility or the lack thereof must be considered for both sexes.
Meiosis and the formation of eggs and sperm
While physiological conditions that impact the structure and function of various reproductive organs can be a source of infertility, the fundamental genetic and age-related factors impacting fertility frequently point to faults in meiosis, the specialized cell division that gives rise to sperm and eggs.
Meiosis comprises a series of distinct steps that produce cells with half the usual number of chromosomes. That way, when an egg is fertilized by a sperm cell, the two half-sets of chromosomes come together to form a full set in the embryo. Understanding the steps of meiosis is a primary focus of Stowers Investigator Scott Hawley, Ph.D.
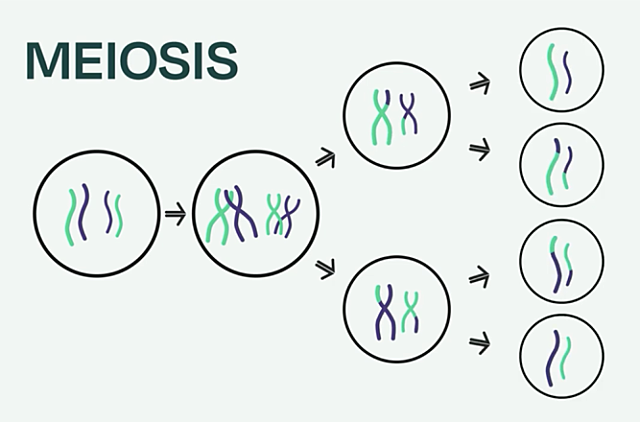
Meiosis involves chromosome duplication followed by two rounds of cell division that give rise to four cells, each with half the number of chromosomes of the starting cell.
First, chromosomes are duplicated and must correctly match up in pairs. Next up, the pairs of maternal and paternal chromosomes literally connect. Then pieces of these chromosomes are swapped between pairs, a process called recombination. In the last steps which occur during two rounds of cell division, the chromosomes must pull apart from each other and separate into four different cells. Each step in the series is like a coordinated dance, until something missteps.

The Hawley Lab developed a 3D model of a large protein structure called the synaptonemal complex which connects chromosomes when they pair up during meiosis.
The Hawley Lab’s research seeks to uncover the molecular and mechanistic underpinnings for how meiosis works. Down the road, insights from this work may have applications for reproductive medicine and personalized healthcare or treatments. “Personalized knowledge provides people with power—power to make informed decisions about their individual circumstances and potentially on the steps needed to overcome infertility,” said Hawley.
Infertility and evolution
Meiosis has been evolutionarily conserved over hundreds of millions of years, perhaps even longer, and across many different species. Infertility is largely due to errors during meiosis that give rise to reproductive cells with incorrect numbers of chromosomes.
Infertility is also linked to the presence of certain rule-breaking “selfish” genes in a population’s genome that decrease its health and fertility. Stowers Associate Investigator SaraH Zanders, Ph.D., studies how selfish genes bend reproductive rules to ensure their persistence in offspring. Generally, inheritance can be viewed like the flip of a coin, but there are exceptions – and a growing awareness that certain genes stack odds in their favor. This occurs across many species at the expense of fertility.
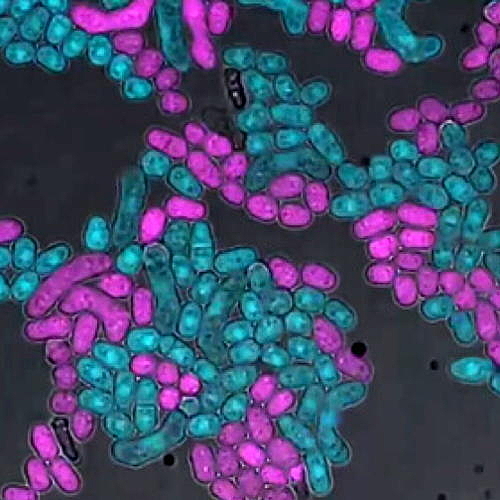
Fission yeast in culture. The Zanders Lab studies fission yeast reproduction and the transmission of selfish genes which can affect fertility.
By using simple single-celled yeast to investigate mechanisms governing selfish gene function and how organisms evolve processes to suppress these, research from the Zanders Lab may provide hope for understanding how these elements can be identified as sources of infertility.
Foundational research at the Stowers Institute is leading to a greater understanding of the many factors governing infertility with the goal of improving health, healthy outcomes, and providing hope for people facing this challenge.
Learn more about the impact of our infertility research at the Stowers Institute here.
In The News

07 January 2026
From the Journal of Cell Science, Investigator Kamena Kostova named a 'Cell Scientist to Watch'
Read Article
#Stowers25: Celebrating 25 Years
06 January 2026
Alejandro Sánchez Alvarado, Ph.D., reflects on a year of discovery, gratitude, and the community that helps support our mission.
Read Article
In The News
01 January 2026
From Science Friday, President and CSO Alejandro Sánchez Alvarado talks about the science of regeneration and the biology lessons we can carry into the new year.
Read Article
