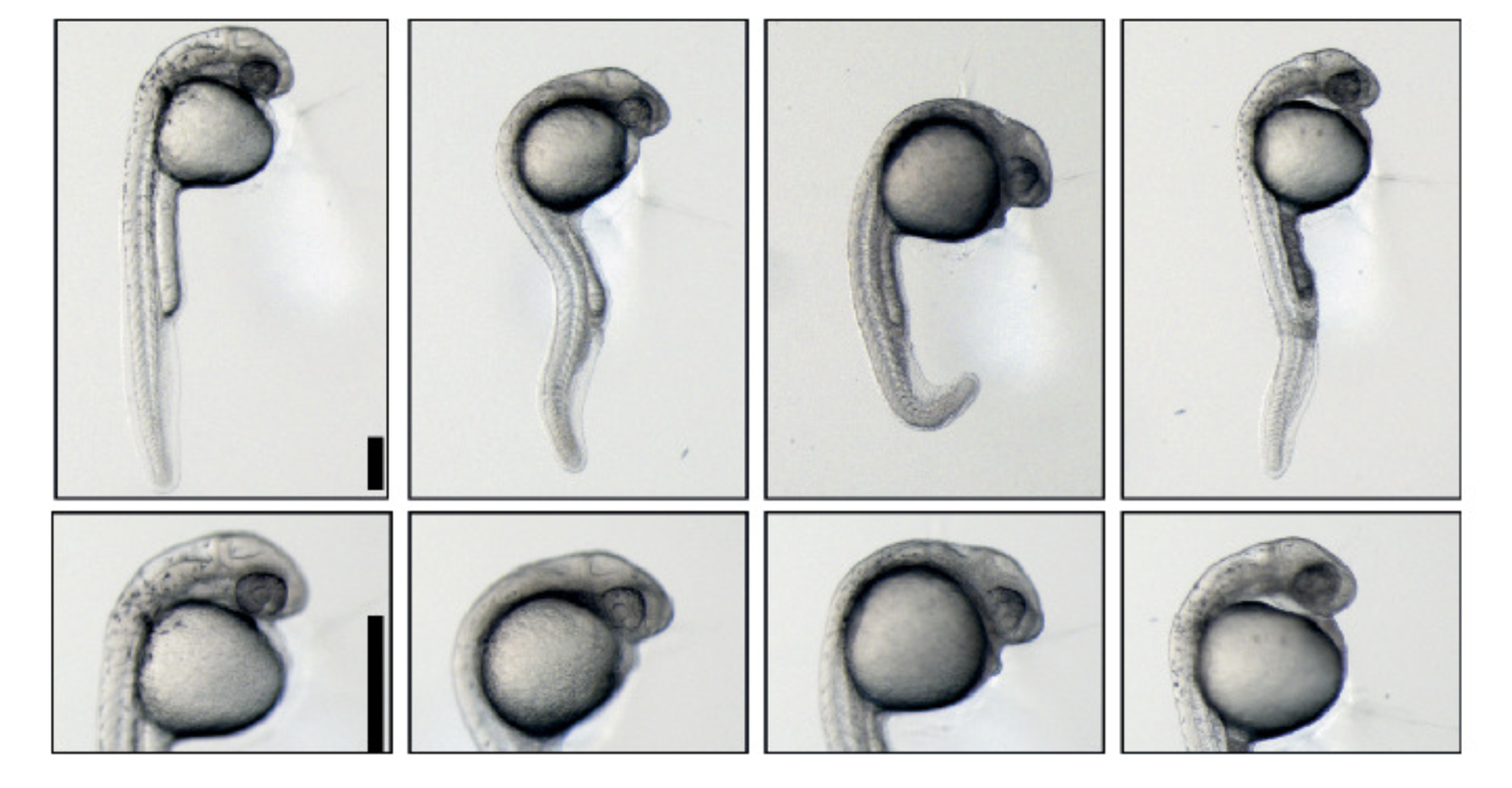
What is most interesting about this work?
Proteins are usually seen as the final functional product of gene expression; however, we discovered that it’s not just about whether certain proteins are present or in what amount, but whether they are “switched on” or “decorated” with chemical modifications. Think of it like a Christmas tree: The tree is the same with or without decorations, but once decorated, it takes on a whole new role and meaning. In the same way, proteins can be decorated with chemical tags that change what they do. Our study showed, for the first time, that this decoration, called phosphorylation, is critical for early development.
What was the most groundbreaking aspect of the study?
Until now, no one knew that phosphorylation — the chemical tags decorating proteins that modify their function — played such an important role at the very start of life. We identified one key “master regulator” protein called a kinase that adds a phosphate “tag” to other proteins. When this master switch is missing, development stalls. We also found that one of the target proteins of the switch must be phosphorylated to activate many other genes.
In short, we discovered a chain of command: One master protein controlled another, which then regulated whole sets of genes. Our next step is to map the phosphorylation process in detail to learn exactly when, where, and which proteins are chemically modified during development.
How did technology and collaboration help?
The discoveries were only possible because of new technologies and strong collaborations. For this work, we teamed up with the Institute’s proteomics facility to create the first large-scale map of protein phosphorylation during early development. At the same time, we were able to test maternal genes one by one. This was done in partnership with the Moreno-Mateos Lab in Seville, Spain — with whom we had previously developed the first CRISPR system in zebrafish capable of directly destroying RNA. By collaborating to combine these two cutting-edge methods — proteomics and CRISPR — our labs finally uncovered a hidden layer of gene control in embryonic development.
Additional authors include Luis Hernández-Huertas, Ph.D., Ismael Moreno-Sánchez, Ph.D., Jesús Crespo-Cuadrado, Ana Vargas-Baco, Gabriel da Silva-Pescador, Ph.D., Ying Zhang, Ph.D., Zhihui Wen, Laurence Florens, Ph.D., and José M. Santos-Pereira.
The Stowers Institute’s portion of this work was supported by the National Institute of General Medical Sciences of the NIH (award: 5R01GM136849-05), the NIH Office of the Director (award: 5R21OD034161-02), and with institutional support from the Stowers Institute for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Full funding information can be found here.