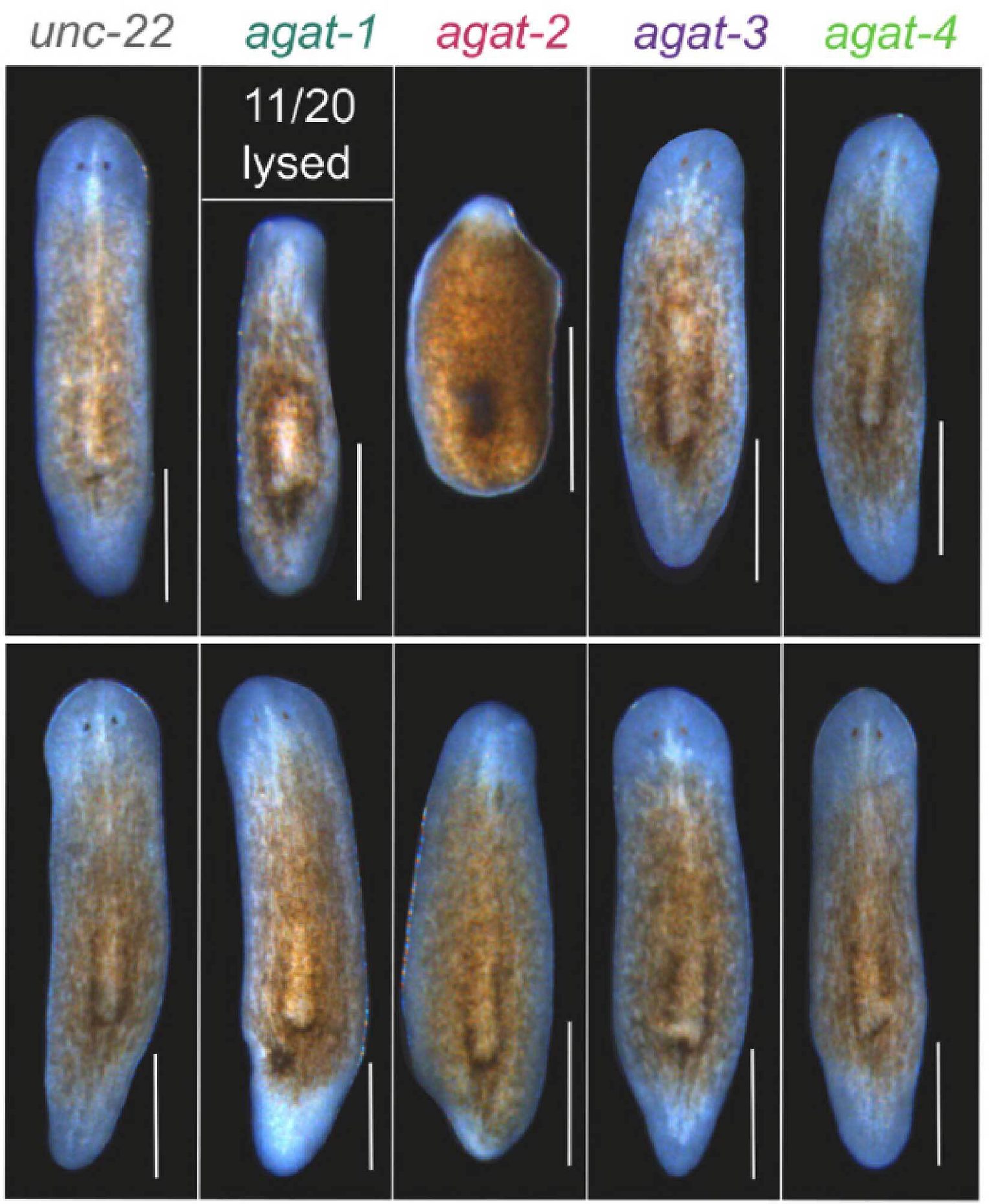
Next, the team screened for factors responsible for the observed degradation, identifying the gene, ZNF574 as a key player in the surveillance pathway. Additionally, the researchers observed that depleting ZNF574 resulted in the accumulation of defective ribosomes, which in turn prevented new ribosomes from assembling.
“We developed a new system to intentionally create faulty ribosomes in human cells, allowing us to investigate how the cell detects and removes these defective parts,” said Kostova. “We discovered a previously unknown quality control system, which helps identify and send faulty ribosomes to the cell's ‘recycling center’.”
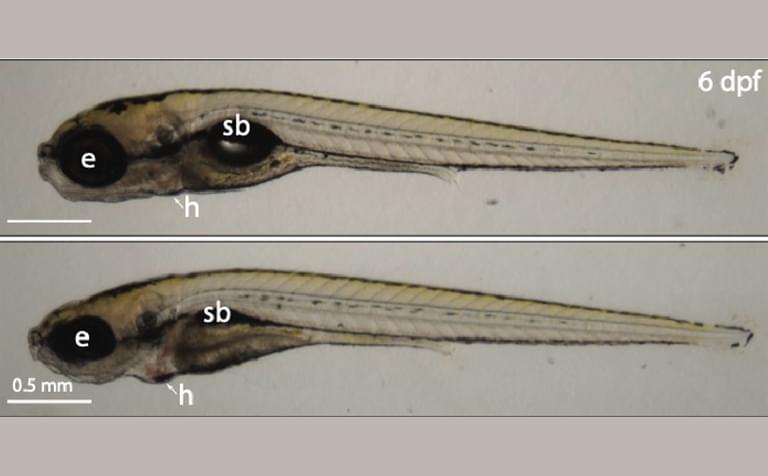
Defects in ribosome production can cause a group of disorders collectively called ribosomopathies, which are characterized by anemia and craniofacial abnormalities. Zebrafish are vertebrate research organisms frequently used to study ribosomopathies. The team discovered that loss of ZNF574 and therefore accumulation of defective ribosomes causes ribosomopathy-like abnormalities in zebrafish.
“Importantly, we found that when this pathway is broken—in both human cells and a zebrafish model—faulty ribosomes build up, leading to problems with cell function and development,” said Kostova.
Ribosome assembly is a fundamental process across the tree of life. Uncovering the Ribosome Assembly Surveillance Pathway quality control mechanism and the protein that governs it may enhance our understanding of both normal development and disease. The new findings may be significant for uncovering novel treatments to address conditions caused by defects in ribosome formation.
Additional authors include Adrian Bothe, Hanna Suh, Carmen Jung, Zachary D. Stolp, Ph.D., Tanushree Ghosh, Ph.D., Liewei L. Yan, Ph.D., Yuming Wang, Michelle Macurak, Amisha Devan, Mary C. McKinney, Ph.D., Tarabryn S. Grismer, Andres V. Reyes, Eric J. Ross, Tianyi Hu, Shou-Ling Xu, Ph.D., and Nenad Ban, Ph.D.
This work was funded by National Institutes of Health (NIH) Directors’ Early Independence Award (award: 5DP5OD028147-03), the Carnegie Endowment Fund, the Jane Coffin Childs Postdoctoral Fellowship, the National Institute of General Medical Sciences of the NIH (awards: F32GM143875, R01GM135706), the Boehringer Ingelheim Fonds Ph.D. fellowship, the Swiss National Science Foundation (SNSF) (award: 310030_212308), the National Center of Excellence in Research RNA and Disease Program of the SNSF (award: 51NF40-205601), and with institutional support from the Stowers Institute for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.