Press Release
23 August 2023
Stowers scientists find evidence of unintended impacts from anti-cancer drugs
Could potentially improve success rate of cancer drug development
Read Article
News
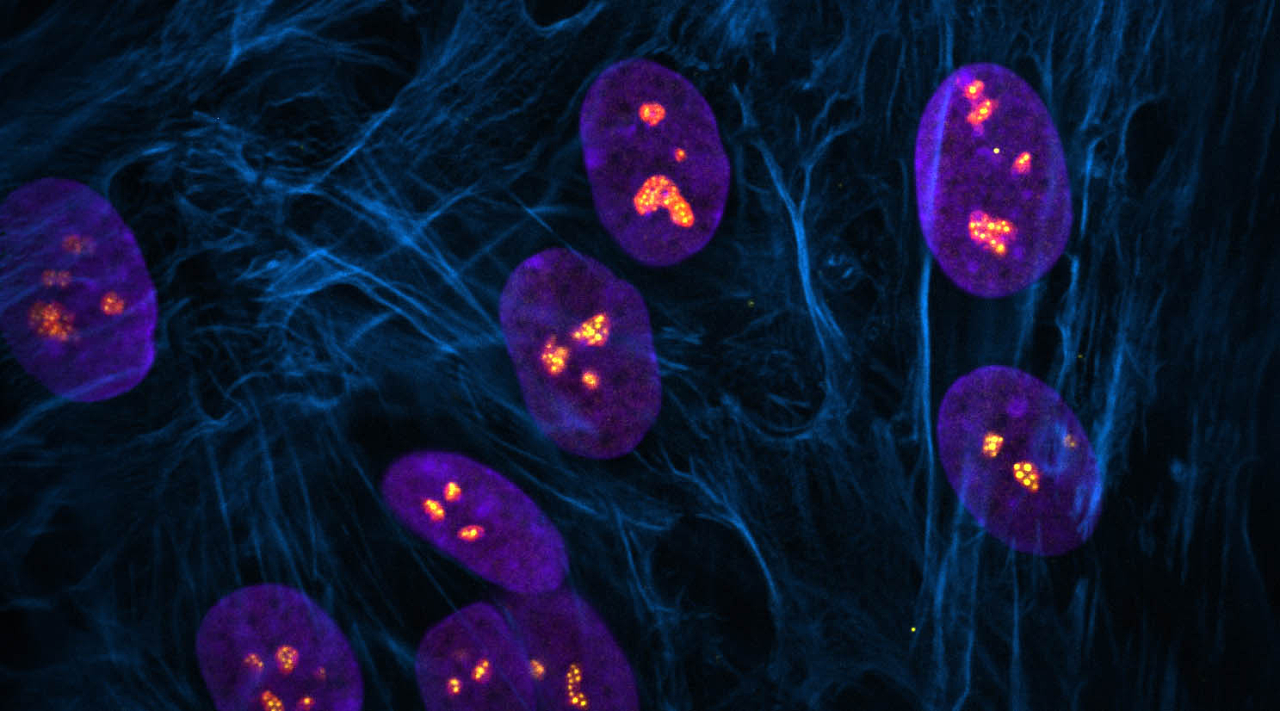
Stowers scientists explore how ribosome biogenesis regulates nucleolar structure during normal development and in the pathogenesis of disease in mice using models of ribosomopathies

Developing mouse embryos at the 8-cell stage (top row: wild type; bottom row: mutant) labeled for compartments of the cell: cell membrane (phalloidin; green), DNA in the cell nucleus (DAPI; blue), and nucleolus where ribosomal RNA is transcribed and assembled into ribosomes (Nucleolin in left column, Treacle in middle column, 5.8S rRNA in right column; magenta). Image credit: Soma Dash, Ph.D., Trainor Lab, Stowers Institute for Medical Research.
Each cell of the body contains many tiny protein factories called ribosomes that are constantly producing all the proteins needed by the cell. Alterations in the precise assembly or regulation of these proverbial well-oiled machines, known as ribosome biogenesis, can disrupt protein production and cause a class of human diseases that affect skeletal and blood development called ribosomopathies, including Treacher Collins Syndrome and Diamond Blackfan Anemia, respectively.
New research from the Stowers Institute for Medical Research published online August 28, 2023, in PLoS Genetics provides insight into the interplay between ribosome biogenesis and their site of assembly, a structure in the cell nucleus called the nucleolus. Previous work in the field has shown that changes in nucleolar structure are associated with the development and progression of ribosomopathies and vice versa. The current study uncovers mechanisms underpinning these associations and their outcomes.
“We believe our findings will have a broad impact on our understanding of fundamental cell biology, and also be of considerable clinical significance potentially providing therapeutic avenues for the prevention of ribosomopathies” said Stowers Investigator Paul Trainor, Ph.D., corresponding author of the study.
The nucleolus has a typical physical structure featuring distinct compartments that correspond to different steps in ribosome biogenesis. Although all ribosomopathies are caused by defects in the global process of ribosome biogenesis, they are quite variable in their characteristics depending on which step of ribosome biogenesis is affected.
Led by Postdoctoral Research Associates Soma Dash, Ph.D., and Maureen Lamb, Ph.D., the research team explored how ribosome biogenesis regulates nucleolar structure during normal development and in the pathogenesis of disease in mice using models of ribosomopathies. They focused on a multi-subunit enzyme called RNA Polymerase I (Pol I) that is responsible for producing ribosomal RNA, a key component of ribosomes.
The researchers identified that changes in nucleolar morphology were caused by alterations in the biophysical properties of compartments of the nucleolus. Changes in the concentration of ribosomal RNA or ribosomal proteins can make nucleolar compartments more sticky or viscous, which then impedes the production of ribosomes. Overall, this work highlights the connection between Pol I function and nucleolar structure during development and how perturbations contribute to disease.
“The nucleolus is a subcellular membrane-less organelle whose structure is intimately tied to its function,” said Dash. “I am intrigued by how this structure is maintained considering the constant influx and outflux of material that takes place in the nucleolus. We were able to solve part of the puzzle by confirming that RNA and particularly, ribosomal RNA is integral to keeping the structure of the nucleolus intact, without which multiple nucleoli collapse into one giant nucleolus in a cell.”
Lamb explained, “Understanding the basic mechanism of how ribosome biogenesis maintains nucleolar structure during development provides important insights into how defects in this process alter nucleolar morphology and lead to the pathogenesis of diseases like ribosomopathies.”
Additional authors include Jeffery J. Lange, Ph.D., Mary McKinney, Ph.D., Dai Tsuchiya, Ph.D., Fengli Guo, Ph.D., Xia Zhao, Ph.D., Timothy J. Corbin, MaryEllen Kirkman, Kym Delventhal, Emma Moore, Sean McKinney, Ph.D., and Rita Shiang, Ph.D.
This work was funded by the American Association for Anatomy, the National Institute for Dental and Craniofacial Research of the National Institutes of Health (NIH) (award: K99DE030972), and institutional support from the Stowers Institute for Medical Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Press Release

23 August 2023
Could potentially improve success rate of cancer drug development
Read Article
News

26 July 2022
Recent studies link severe craniofacial disorders to disruptions during development
Read Article
News

12 August 2021
Kristin Watt, Curtis Bacon, Jasmin Camacho receive notification of funding.
Read Article
